Carboline carboxylic acid-tetrapeptide conjugate and synthesis method as well as medical application thereof
A carboline carboxylic acid and peptide coupling technology, applied in the field of biomedicine, can solve the problems of low solubility and bioavailability, and achieve excellent antithrombotic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
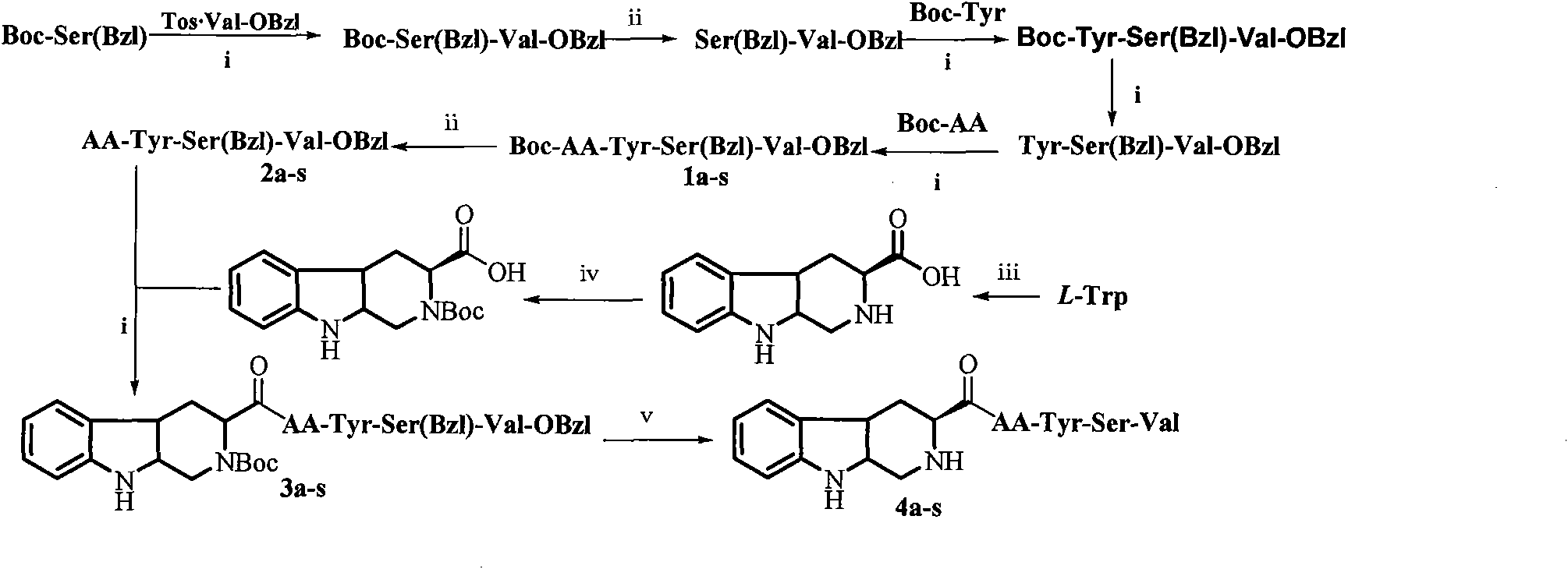
[0016] Example 1 Preparation of 3S-1,2,3,4-tetrahydro-β-carboline-3-acyl-Val-Tyr-Ser-Val (4a) 1) 3S-1,2,3,4-tetra Preparation of Hydrogen-β-carboline-3-carboxylic Acid
[0017] Put 400ml of water in a 500ml round bottom flask, slowly add 0.2ml of concentrated sulfuric acid and shake well. Then add 5.0g (24.5mmol) L-tryptophan, ultrasonically shake until the L-tryptophan is completely dissolved, add 10ml of formaldehyde with a concentration of 35% to the above mixture, stir the reaction, and the TLC plate shows L-tryptophan The raw material point disappeared, and slowly added ammonia water to the reaction solution to pH = 6.0, let it stand for 0.5 hours, filtered under reduced pressure to obtain a white solid, dried and weighed 5.01g, yield: 95.0%, m.p: 228-230°C, ESI -MS(m / z): 217[M+H] +
[0018] 2) Preparation of N-tert-butyryl-3S-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid
[0019] Under ice bath conditions, 1.0g (4.63mmol) 3S-1,2,3,4-tetrahydro-β-carboline-3-carbox...
Embodiment 2
[0038] Example 2 Preparation of 3S-1,2,3,4-tetrahydro-β-carboline-3-acyl-Tyr-Tyr-Ser-Val (4b)
[0039] 1) Preparation of Boc-Tyr-Tyr-Ser(Bzl)-Val-OBzl(1b)
[0040] Using the method for preparing Boc-Tyr-Ser(Bzl)-Val-OBzl, with 1.238g (2.26mmol) Tyr-Ser(Bzl)-Val-OBzl and 0.762g (2.71mmol) Boc-Tyr, the title compound 1.166 was obtained g, colorless solid, yield 63.63%, TLC (chloroform:methanol=20:1, Rf=0.26) m.p: 86.9~88.5°C, ESI-MS(m / z): 812[M+H] + .
[0041] 2) Preparation of Tyr-Tyr-Ser(Bzl)-Val-OBzl(2b)
[0042] Using the method for preparing Ser(Bzl)-Val-OBzl, with 1.098g (1.35mmol) Boc-Tyr-Tyr-Ser(Bzl)-Val-OBzl and 5.5ml hydrogen chloride-ethyl acetate (4N), the title compound was obtained 0.960g, yellow oil, yield 99.79%. ESI-MS(m / z): 712[M+H] +
[0043] 3) Preparation of Boc-3S-1,2,3,4-tetrahydro-β-carboline-3-acyl-Tyr-Tyr-Ser(Bzl)-Val-OBzl (3b)
[0044] Using the method for preparing Boc-Tyr-Ser(Bzl)-Val-OBzl, with 0.960g (1.35mmol) Tyr-Tyr-Ser(Bzl)-Val-OBzl an...
Embodiment 3
[0047] Example 3 Preparation of 3S-1,2,3,4-tetrahydro-β-carboline-3-acyl-Ala-Tyr-Ser-Val (4c)
[0048] 1) Preparation of Boc-Ala-Tyr-Ser(Bzl)-Val-OBzl(1c)
[0049] Using the method for preparing Boc-Tyr-Ser(Bzl)-Val-OBzl, with 1.260g (2.30mmol) Tyr-Ser(Bzl)-Val-OBzl and 0.522g (2.76mmol) Boc-Ala, the title compound 1.169 was obtained g, colorless solid, yield 70.69%, TLC (chloroform:methanol=30:1, Rf=0.27) m.p: 78.9~80.9°C, ESI-MS(m / z): 720[M+H] + .
[0050] 2) Preparation of Ala-Tyr-Ser(Bzl)-Val-OBzl(2c)
[0051] The title compound was obtained by preparing Ser(Bzl)-Val-OBzl with 0.929g (1.29mmol) Boc-Ala-Tyr-Ser(Bzl)-Val-OBzl and 3.7ml hydrogen chloride-ethyl acetate (4N) 0.7988g, yellow oil, yield 99.75%. ESI-MS(m / z): 620[M+H] +
[0052] 3) Preparation of Boc-3S-1,2,3,4-tetrahydro-β-carboline-3-acyl-Ala-Tyr-Ser(Bzl)-Val-OBzl(3c)
[0053] Using the method for preparing Boc-Tyr-Ser(Bzl)-Val-OBzl, with 0.798g (1.29mmol) Ala-Tyr-Ser(Bzl)-Val-OBzl and 0.493g (1.55mmol) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com