PEG-PLGA nanoparticle carrying recombinant human vascular endothelial inhibitor and preparation method thereof
A PEG-PLGA90.2%-99%, vascular endothelial technology, applied in the direction of drug combination, pharmaceutical formula, medical preparations of non-active ingredients, etc., can solve the problem of protein drugs with large molecular weight, difficult to wrap nanoparticle carriers, and carrier materials Large molecular weight and other problems, to achieve the effect of high drug loading, prolonged residence time, and good sustained release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
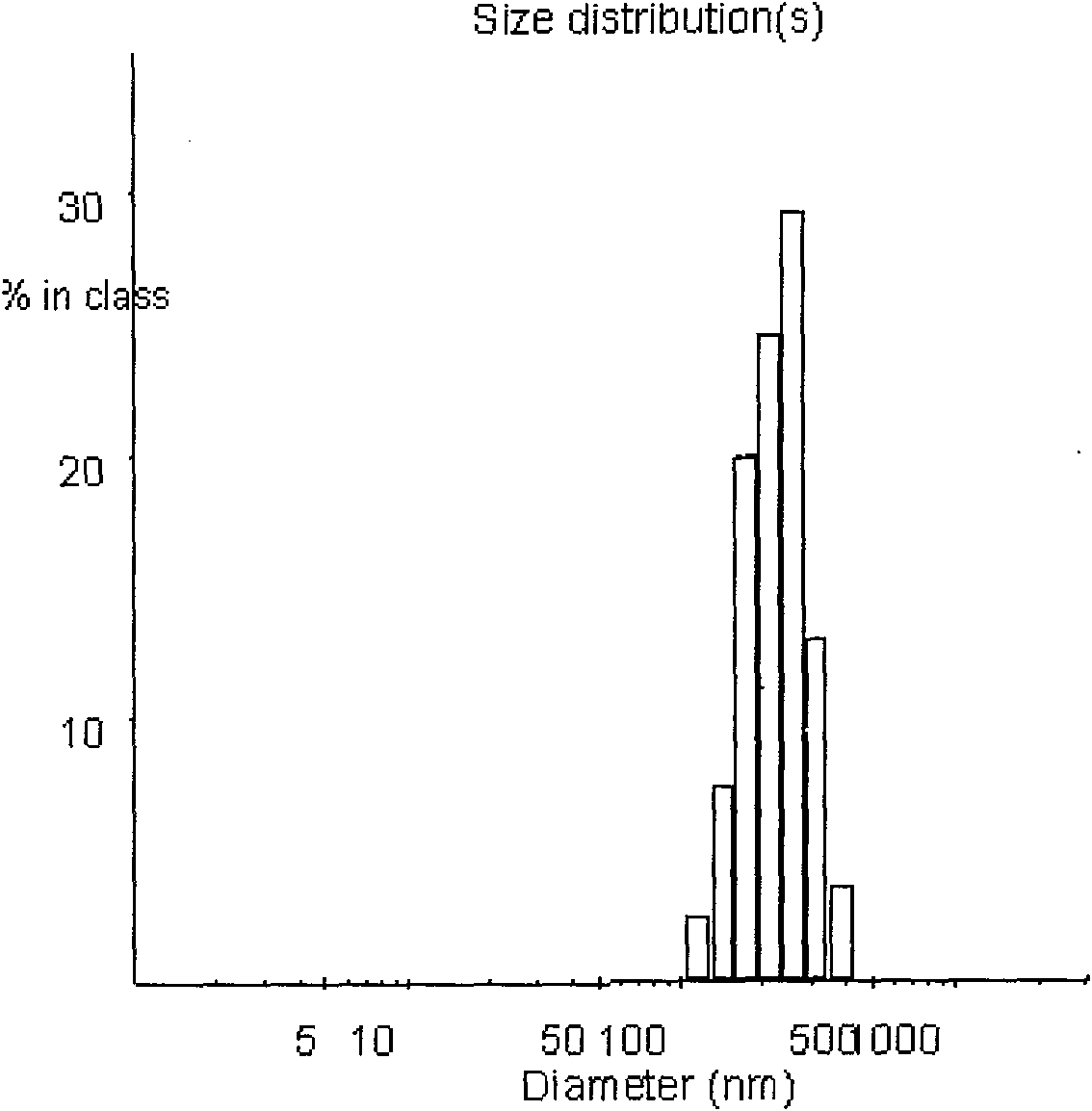
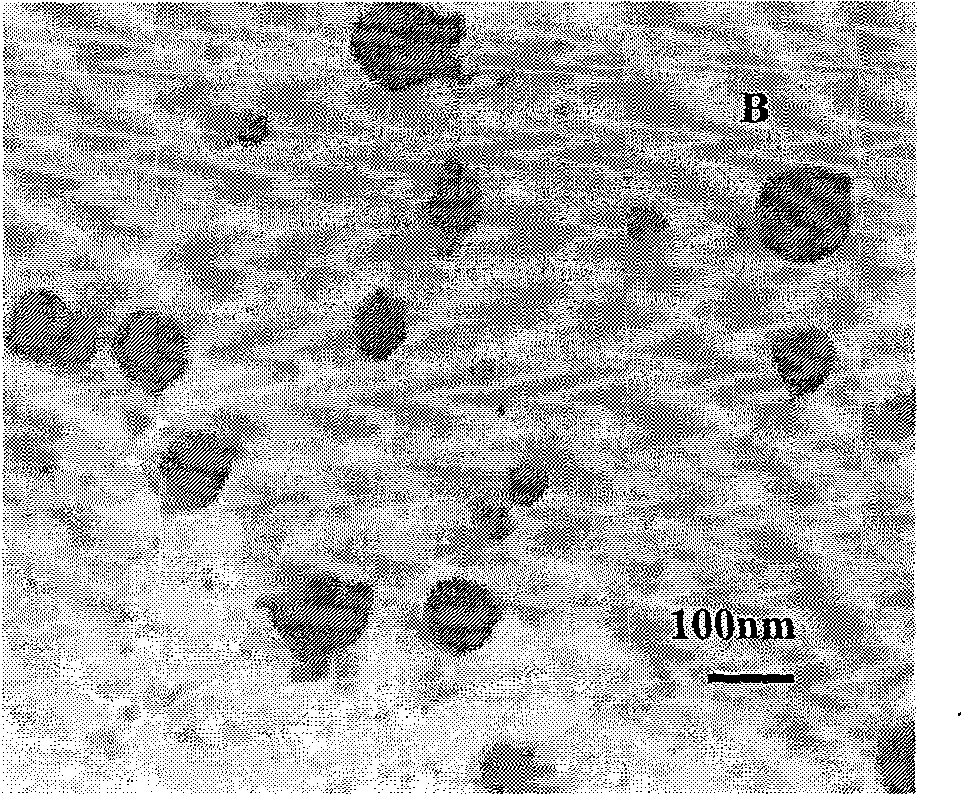
[0030] Accurately weigh 5 mg of PEG-PLGA, add to 1 ml of dichloromethane and dissolve to form the organic phase. Take 50ul of endostar aqueous solution with a concentration of 10mg / ml as the water phase. Add the Endostar solution to the organic phase and vortex mix at 2800rpm, and form colostrum after homogenization. Weigh polyvinyl alcohol (PVA) and add it into double-distilled water to make it fully dissolved, adjust the concentration to 0.1%, and form the external water phase. The volume ratio of the inner water phase to the outer water phase is 1:4. Immediately add the prepared colostrum into the external water phase, shear at 25000rpm for 60s at high speed, pour the obtained emulsion into 10ml of 0.1% polyvinyl alcohol solution, stir and evaporate the organic solvent for 4 to 5 hours with magnetic stirring, and completely volatilize the dichloromethane , to obtain a colloidal solution of PEG-PLGA nanoparticles. The average particle size is 109nm, and the encapsulation ...
Embodiment 2
[0032] Accurately weigh 10 mg of PEG-PLGA, add to 1 ml of dichloromethane and dissolve to form the organic phase. Take 50ul of endostar aqueous solution with a concentration of 10mg / ml as the water phase. Add the Endostar solution to the organic phase and vortex mix at 2800rpm, and form colostrum after homogenization. Weigh polyvinyl alcohol (PVA) and add it into double-distilled water to make it fully dissolved, and adjust its concentration to 0.25% to form the external water phase. The volume ratio of the inner water phase to the outer water phase is 1:4. Immediately add the prepared colostrum into the external water phase, shear at 20,000rpm for 60s at high speed, pour the obtained emulsion into 10ml of 0.25% polyvinyl alcohol solution, and evaporate the organic solvent with magnetic stirring for 4-5 hours to completely volatilize the dichloromethane , to obtain a colloidal solution of PEG-PLGA nanoparticles. The average particle size is 198nm, and the encapsulation effi...
Embodiment 3
[0034]Accurately weigh 18 mg of PEG-PLGA, add it to 1 ml of dichloromethane and dissolve it to form the organic phase. Take 200ul of endostar aqueous solution with a concentration of 10mg / ml as the water phase. Add the Endostar solution to the organic phase and vortex mix at 2000rpm, and form colostrum after homogenization. Weigh polyvinyl alcohol (PVA) and add it into double-distilled water to make it fully dissolved, and adjust its concentration to 0.25% to form the external water phase. The volume ratio of the inner water phase to the outer water phase is 1:4. Immediately add the prepared colostrum into the external water phase, shear at 20,000rpm for 60s at high speed, pour the obtained emulsion into 10ml of 0.25% polyvinyl alcohol solution, and evaporate the organic solvent with magnetic stirring for 4-5 hours to completely volatilize the dichloromethane , to obtain a colloidal solution of PEG-PLGA nanoparticles. The average particle diameter is 253nm, and the encapsul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com