Preparation method of 4-phosphoric acid erythrose
A technology of red fresh sugar phosphate and fructose phosphate, which is applied in the field of biotechnology applications, can solve the problems of high requirements for reaction equipment, complex product components, and low yield, and achieve low requirements for reaction equipment, high product yield, and high yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Cloning of histoplasma dermatitis (ATCC 18188) transketolase gene (tktA)
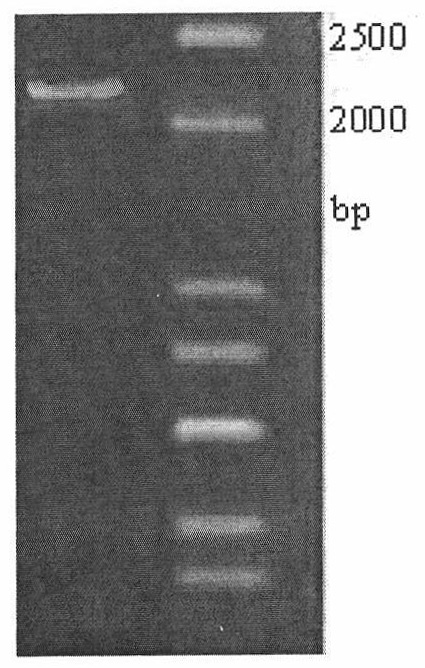
[0025] Using 0.5μg of Histioplasma dermatitis genomic DNA as the PCR reaction template, the forward primer tktA-F was designed according to the sequence of transketolase (as shown in SEQ ID NO. The primer tktA-R is 5'-TTGCCTCGAGAATCTACTAAACGACCTTCCGC-3', and the italicized letters are the restriction sites BamHI and XhoI, respectively. The design of the 3'primer ensures that the expression product contains 6 histidine tags. The PCR reaction was carried out in a total volume of 50 μL. The reaction conditions were denaturation at 94°C for 5 minutes and then cycle, then denaturation at 94°C for 50 seconds, annealing at 56°C for 1 minute, and extension at 72°C for 2 minutes. After a total of 30 cycles, the reaction was extended at 72°C for 10 minutes. Take 5μL of PCR amplification product for agarose gel electrophoresis verification ( figure 1 ). Take 100μL of PCR product for agarose gel ele...
Embodiment 2
[0026] Example 2 Construction of tktA expression vector and its expression in E. coli JM109
[0027] 1. Construction of tktA gene: The PCR product in Example 1 was recovered by gel. The purified PCR product and pET-22b(+) vector were digested with BamH I and XhoI respectively, and recovered by the gel recovery kit, then ligated (16°C, 16h), transformed into E. coli DH5a competent cells, and selected Positive clones, after extracting the plasmid, use BamHI and XhoI for restriction analysis ( figure 2 ), DNA sequencing identification is performed after verification. Construction of pET-22b(+)-tktA expression vector.
[0028] 2. Expression of tktA gene: Transform E. coli JM109 competent cells with the pET-22b(+)-tktA expression plasmid verified by sequencing; pick positive clones, inoculate them in LB medium containing kanamycin, and shake at 37°C Cultivate OD 600 When it reaches 0.6-0.8, add IPTG with a final concentration of 1mmol / L and induce 3h at 37℃. Inoculate the transforme...
Embodiment 3
[0029] Example 3 Separation and purification of TK and detection of enzyme activity
[0030] 1. Purification of Histoplasma dermatitis transketolase: The fermentation broth of Example 2 was centrifuged to collect the bacteria, and the bacteria were suspended in a 50mmol / L, pH 8.0 Tris-HCl (containing 1mmol / L) buffer (wet bacteria) The ratio of the buffer solution is 1g:5mL), the bacterial cells are broken by ultrasonic in an ice bath, and the supernatant is collected after centrifugation. Pack the column with NTA medium, wash with 2 times the column volume of deionized water, and add NiSO 4 The solution was transferred to NTA medium to saturate the binding degree, and the column was washed with 5 column volumes of NAT-1 buffer (20mM Tris-HCl pH7.9, 0.5M NaCl). Add the supernatant obtained above to Ni 2+ -NTA resin column, after adding 3 to 5 times repeatedly, wash the medium with 5 column volume of NAT-2 buffer (NAT-0 buffer contains 80mmol / L imidazole) to remove impurities, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com