New aurantiamarin synthesizing technique
A new hesperidin and synthetic process technology, applied in the direction of organic compound/hydride/coordination complex catalyst, preparation of sugar derivatives, sugar derivatives, etc., can solve complex operation, loss of industrial production, and long production cycle and other problems, to achieve the effect of simple synthesis conditions, low production cost, and fast reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
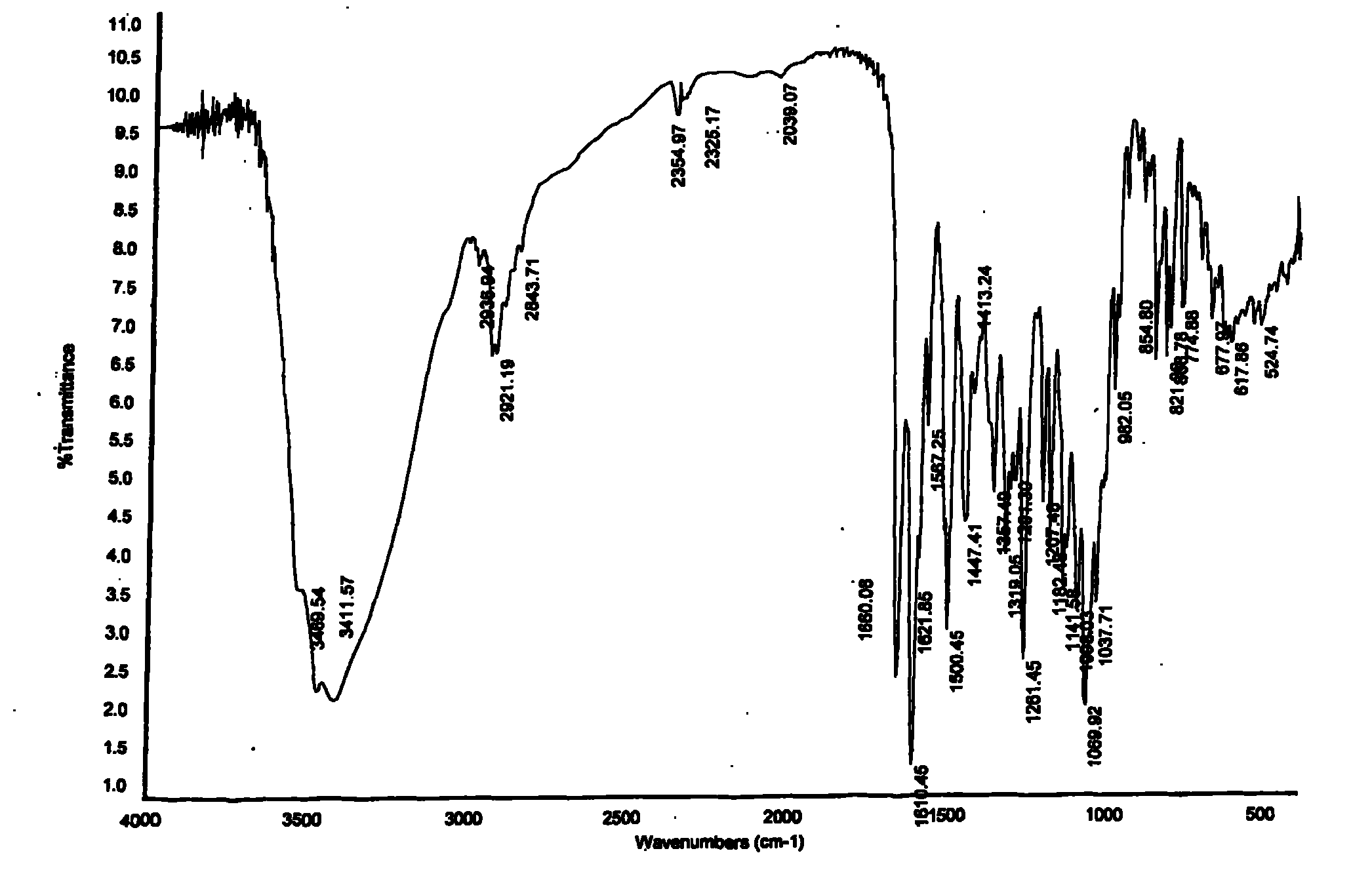
Image
Examples
Embodiment 1
[0018] The operating method of this example synthetic neohesperidin is carried out according to the following two steps:
[0019] Synthesis of root bark acetophenone-4′-β-neohesperidoside: Dissolve 6.0g of sodium hydroxide in 50mL of tap water with stirring, then slowly add 4.5g of naringin into the alkaline solution with stirring , after stirring completely to dissolve, start to heat up to reflux at 100°C, count from the start of reflux, stop heating after reflux and stirring for 1.5 hours, cool to below 30°C, slowly add concentrated hydrochloric acid to neutralize to a pH value of about 6.5, and a yellow solid precipitates. Heat again to above 70°C to dissolve all the solids, stop heating, cool naturally, let stand for 2 hours, then filter through the Buchner funnel, vacuum dry at 60°C to obtain 2.5g of yellow root bark acetophenone-4'- β-Neohesperidoside, yield 68%.
[0020] Synthesis of neohesperidin: Add 2.0g root bark acetophenone-4'-β-neohesperidoside into 12mL85% etha...
Embodiment 2
[0022] The operating method of this example synthetic neohesperidin is carried out according to the following two steps:
[0023] Synthesis of root bark acetophenone-4′-β-neohesperidoside: 3.5kg of sodium hydroxide was dissolved in 30L of tap water under stirring, and then 2.8kg of naringin was slowly added to the alkaline solution under stirring , after stirring to dissolve completely, start heating and heating to 100°C to reflux, count from the start of reflux, reflux at 100°C and stir for 2 hours, then stop heating, cool to below 30°C, slowly add concentrated hydrochloric acid to neutralize to a pH value of about 6.5, and yellow precipitates solid. Heating again to above 70°C to dissolve all the solids, then stop heating, cool naturally, let stand for 2 hours, then filter through Buchner funnel, vacuum dry at 60°C to obtain 1.6kg of yellow root bark acetophenone-4'- β-Neohesperidoside, the yield is 70%.
[0024] Synthesis of neohesperidin: Add 1.5kg root bark acetophenone...
Embodiment 3
[0026] The operating method of this example synthetic neohesperidin is carried out according to the following two steps:
[0027] Synthesis of root bark acetophenone-4'-β-neohesperidoside: 200kg of sodium hydroxide was dissolved in 1700L tap water under stirring, then 150kg of naringin was slowly added to the alkaline solution under stirring, and stirred After completely dissolving, start heating to reflux at 100°C, count from the beginning of reflux, reflux at 100°C and stir for 2 hours, then stop heating, cool to below 30°C, slowly add concentrated hydrochloric acid to neutralize to a pH value of about 6.5, and a yellow solid precipitates. Heat again to above 70°C to dissolve all the solids, stop heating, cool naturally, let stand for 2 hours, then centrifuge and filter, and vacuum dry at 60°C to obtain 85.5kg of yellow root bark acetophenone-4'-β- Neohesperidoside, yield 69%.
[0028] Synthesis of neohesperidin: Add 85kg root bark acetophenone-4'-β-neohesperidoside into 620L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com