Human IL-15 subtype protein and preparation method and application thereof
A protein and sequence technology, applied in the field of human IL-15 subtype protein preparation, can solve problems such as limiting the application of IL-15 subtype protein and prone to allergic reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
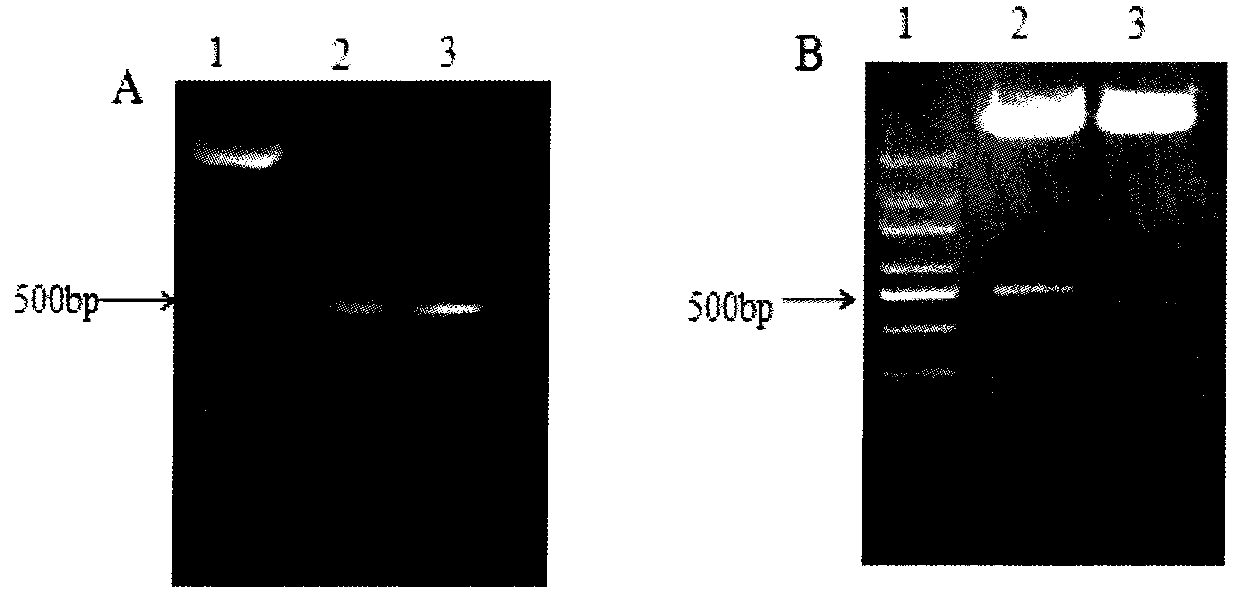
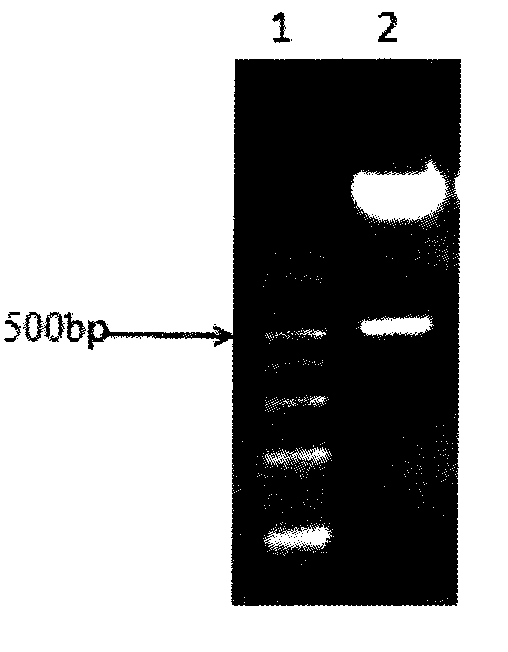
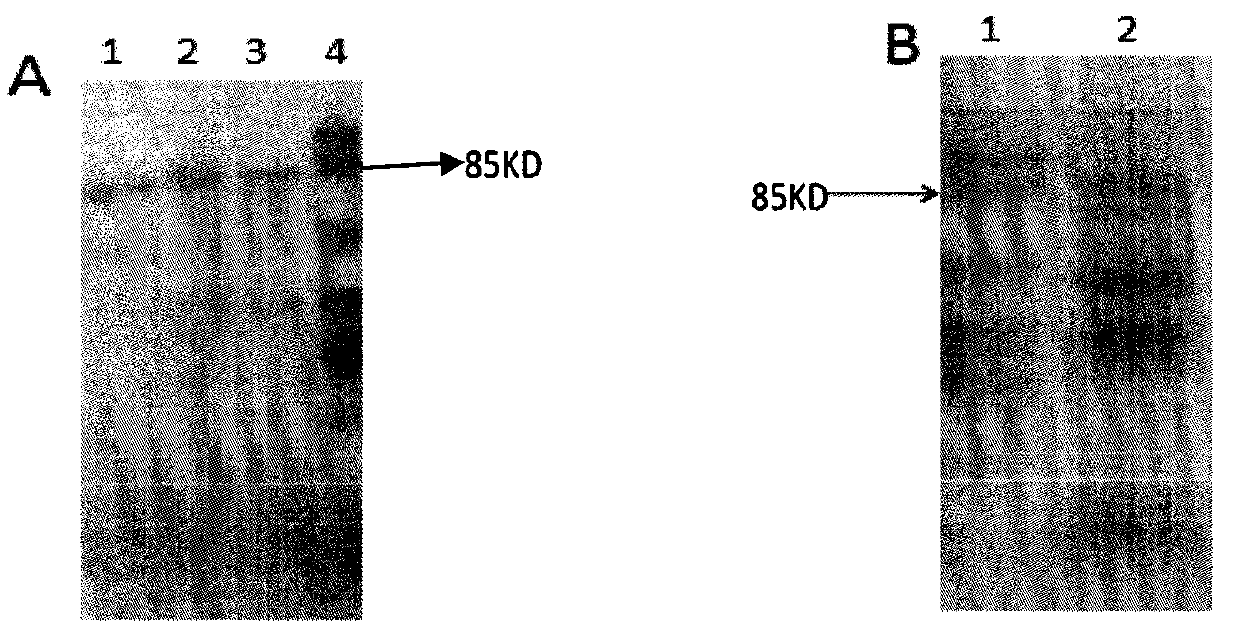
[0061] Example 1, preparation of purified functional untagged human IL-15 subtype protein
[0062] 1. Extraction of total cellular RNA
[0063] Take human peripheral blood cells, prepare peripheral blood mononuclear cell suspension, use 0.1 μg / ml LPS to act for 72 hours to stimulate peripheral blood mononuclear cell PBMC, obtain stimulated peripheral blood mononuclear cell suspension PBMC, add 1ml of lysing solution Trizol, repeat Mix by pipetting, dissolve the cells in Trizol, incubate the sample at room temperature for 5min to lyse the cells; add 0.2ml chloroform, mix vigorously for 15s, incubate the sample at room temperature for 3-5min, centrifuge at 12000g for 15min at 4°C; transfer the upper colorless aqueous phase after centrifugation To the EP tube, add 0.5ml of isopropanol, mix well, ice bath for 10min; centrifuge at 12000g for 10min at 4°C, carefully remove the supernatant; add 1ml of 75% ethanol, mix by inverting, centrifuge at 7500g for 5min at 4°C, wash twice Dis...
Embodiment 2
[0144] The concentration of LPS used in step 1 in this embodiment is 10 μg / ml, and the action time is 24 hours. The rest of the reagents and operating steps are exactly the same.
Embodiment 3
[0146] The concentration of LPS used in step 1 of this example is 20 μg / ml, and the action time is 24 hours; the rest of the reagents and operation steps are exactly the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com