Synthetic method of polynitrogen azole zinc/cadmium framework material
A frame material, polyazole zinc technology, applied in the direction of zinc organic compounds, cadmium organic compounds, etc., can solve the problems of high cost, difficulty in activation, low reaction yield, etc., and achieve rapid reaction, accelerated reaction speed, and post-processing of products simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
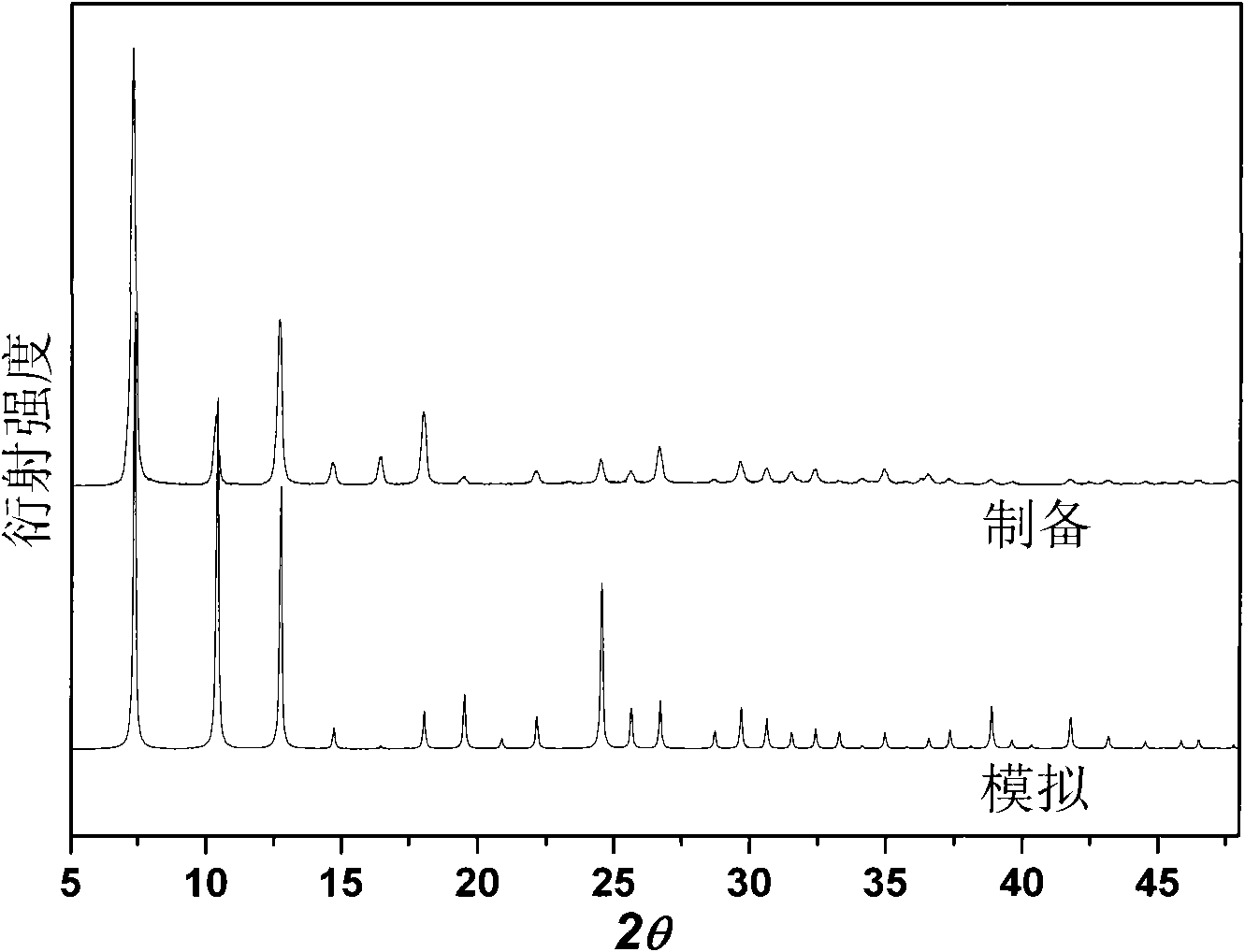
[0035] The room temperature efficient preparation of a small amount of MAF-4 of embodiment 1
[0036] In a 10mL ground-mouth round bottom flask, add 99.4mg Zn(OH) 2 Or 81.4mg ZnO (1.0mmol), 164.2mg Hmim (2.0mmol), 5.0mL reaction solution, capped, stirred at room temperature for 24h and then filtered. The filter cake was washed three times with methanol and dried at 120°C to obtain the product with a yield of about 95%. The reaction solution consisted of 4.0 mL of methanol and 1.0 mL of 25 wt% ammonia water during the initial synthesis. The filtrate obtained after each synthesis can be directly used as the reaction solution for the next synthesis, and can be recycled at least 5 times without reducing the purity and yield of the product.
Embodiment 2
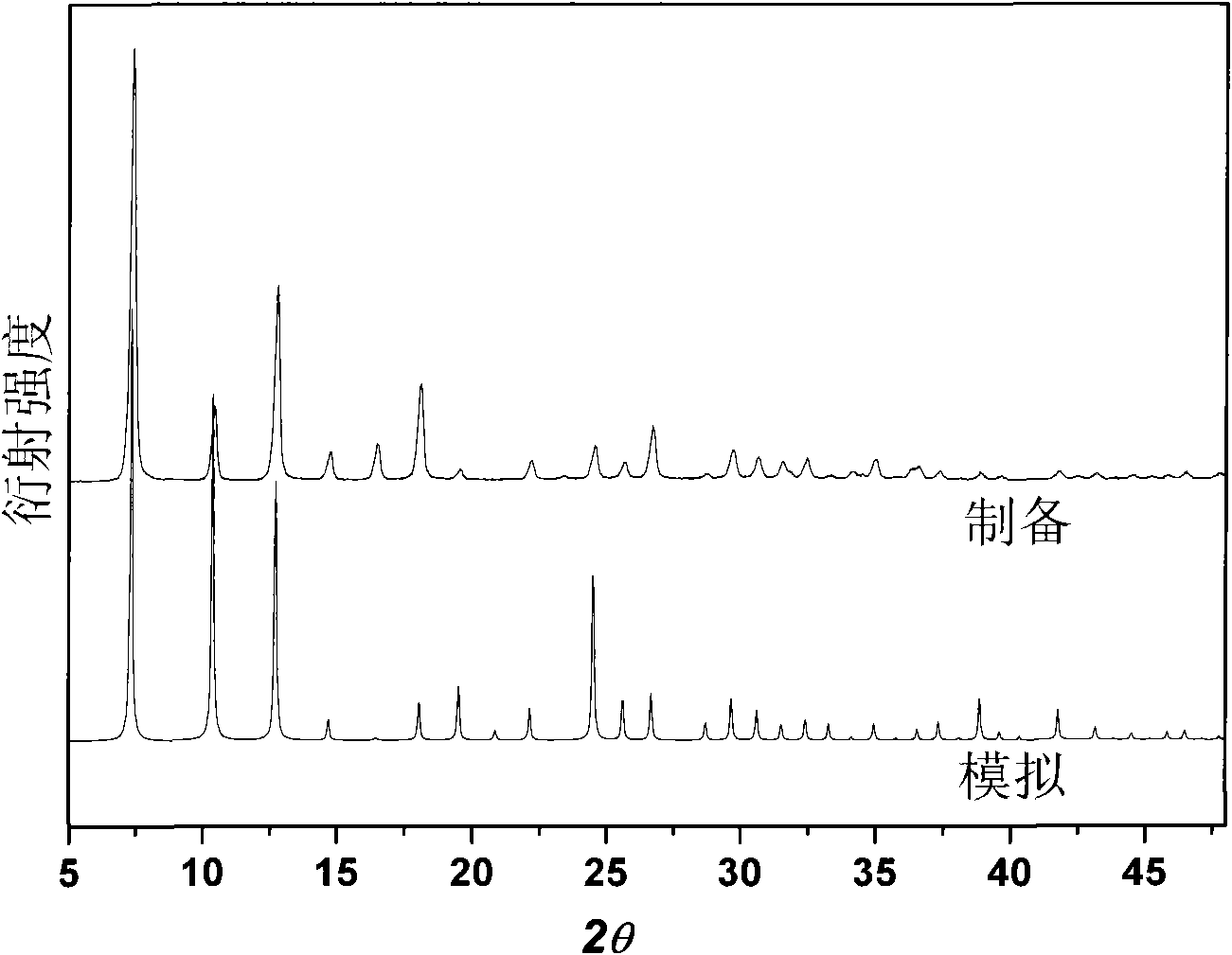
[0037] Efficient preparation at room temperature of a large amount of MAF-4 of embodiment 2
[0038] In a 1000mL ground-mouth round bottom flask, add 99.4g Zn(OH) 2 Or 81.4g ZnO (1.0mol), 164.2g Hmim (2.0mol), 500mL reaction solution, cover, stir vigorously at room temperature for 24h, and then filter. The filter cake was washed three times with methanol and dried at 120°C to obtain the product with a yield of about 95%. The reaction solution consisted of 400mL of methanol and 100mL of 25wt% ammonia water during initial synthesis. The filtrate obtained after each synthesis can be directly used as the reaction solution for the next synthesis, and can be recycled at least 5 times without reducing the purity and yield of the product.
Embodiment 3
[0039] The microwave heating fast preparation of embodiment 3MAF-4
[0040] Add 99.4mg Zn(OH) to a 60mL microwave reactor with Teflon substrate 2 Or 81.4mg ZnO (1.0mmol), 164.2mg Hmim (2.0mmol), 5.0mL reaction solution, stirred at room temperature for a few minutes, sealed, microwave heated to 40°C for 1.5h, cooled to room temperature and filtered. The filter cake was washed three times with methanol and dried at 120°C to obtain the product with a yield of about 95%. The reaction solution consisted of 4.0 mL of methanol and 1.0 mL of 25 wt% ammonia water during the initial synthesis. The filtrate obtained after each synthesis can be directly used as the reaction solution for the next synthesis, and can be recycled at least 5 times without reducing the purity and yield of the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com