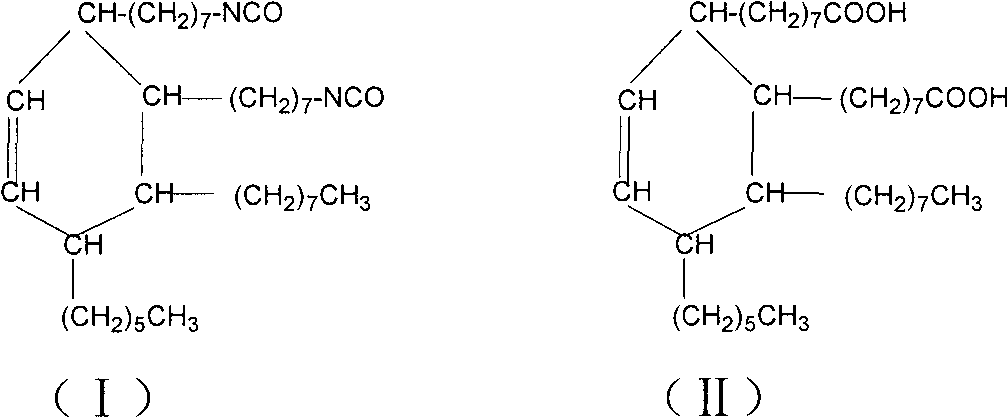
Synthesis method of dimer(fatty acid)yl diisocyanate
A technology for dimerization of fatty acid diisocyanate and synthesis method, which is applied in the preparation of carboxylic acid nitrogen-containing derivatives, organic chemistry, etc., can solve problems such as low yield, and achieve the effect of high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
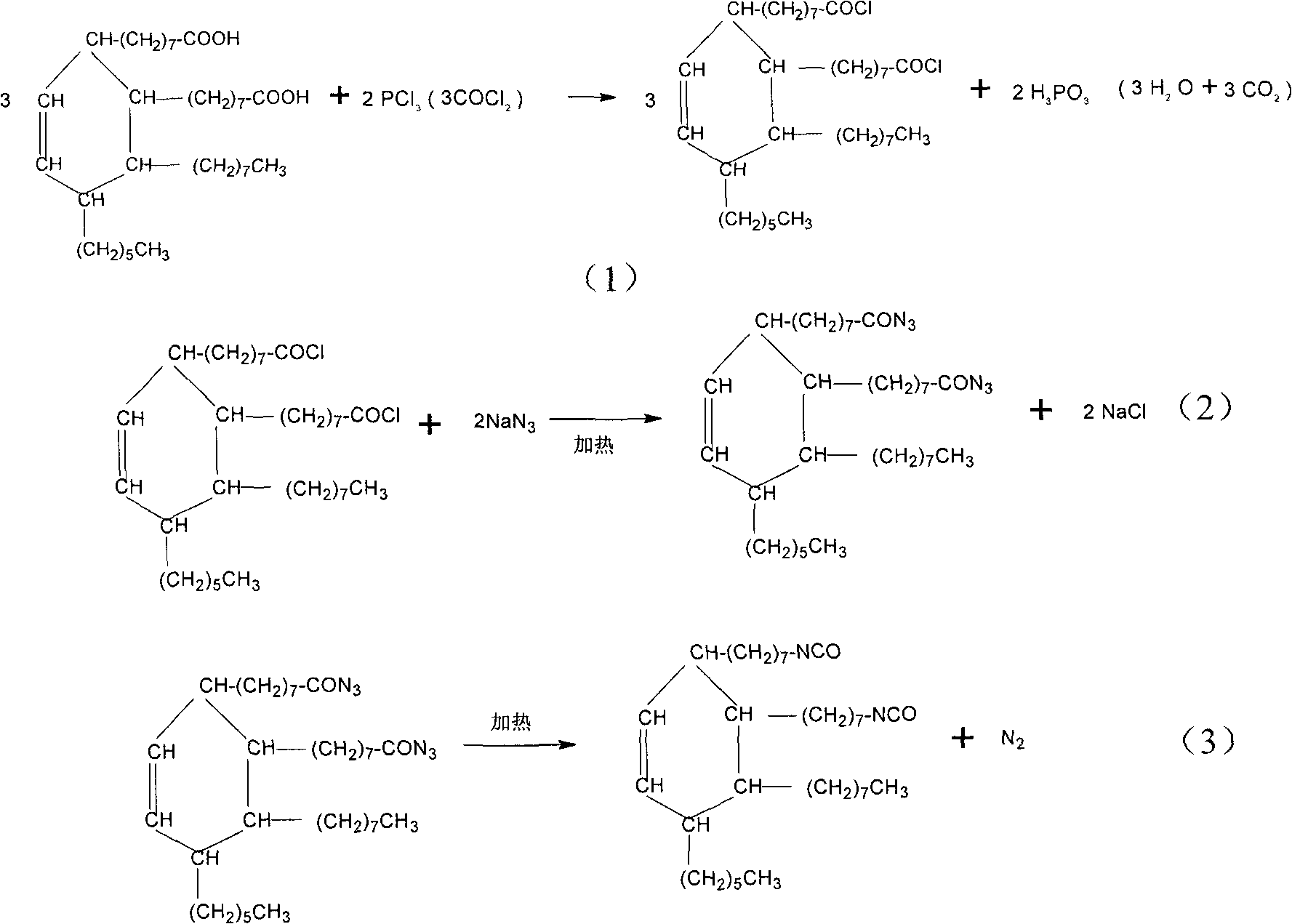
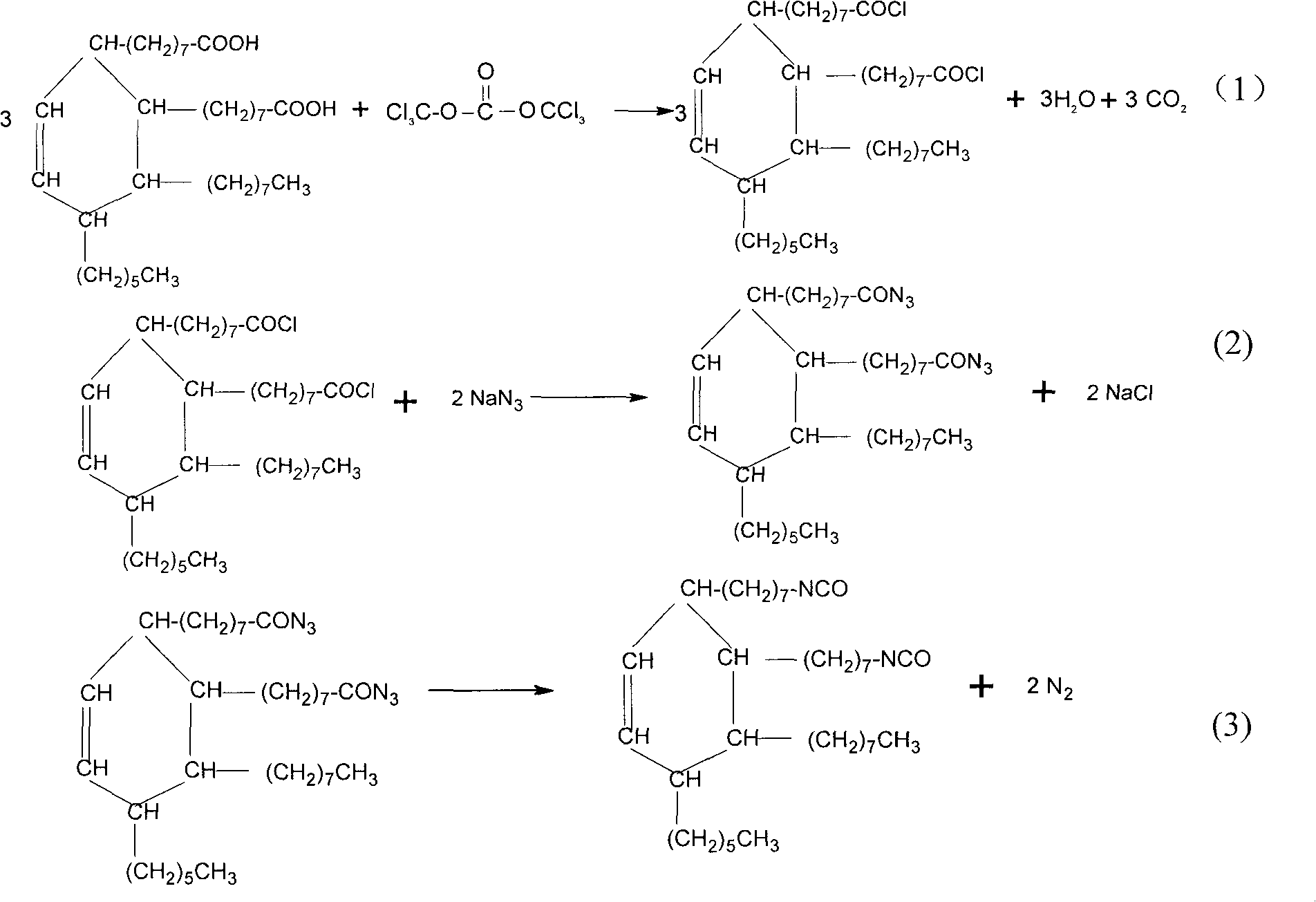
[0018] (1) 400ml of toluene solution containing 112.40g (0.20mol) of dimerized tall oleic acid and 5g of catalyst dimethylformamide were respectively added to a 1000ml reaction flask with a thermometer and a dropping funnel, and stirred at a temperature of 70°C. 150ml of toluene solution dissolved with 29.70g (0.10mol) of bis(trichloromethyl) carbonate was added dropwise under the state, and the reaction was continued for 1 hour after the dropwise addition, cooled to room temperature, filtered, and after toluene was evaporated, 114.76g of dichloromethane was obtained. Polytall oleic acid chloride;
[0019] (2) 54.58g (0.42mol) of sodium azide and 120ml of deionized water were respectively added to a 1000ml reaction flask with a thermometer placed on an ice-water bath, and the solution was added dropwise at a temperature of 10°C under stirring. There is a 200 ml acetone solution of 114.76 g of dimerized tall oil acid chloride obtained in step (1), after the dropwise addition of...
Embodiment 2
[0032] (1) The method is the same as in Example 1, except that the reaction temperature in step (1) is 80° C., and the bis (trichloromethyl) carbonate is 19.80 g (0.067 mol) to obtain 115.12 g of dimerized tall oil acid. acid chloride;
[0033] (2) method is with embodiment 1, and the difference is that the sodium azide in step (2) is 49.78g (0.394mol);
[0034] (3) The method is the same as that in Example 1, except that the reaction temperature in step (3) is 70° C. to obtain 81.12 g of a brown-red transparent oily liquid with a total yield of 72.95%.
Embodiment 3
[0036] (1) The method is the same as in Example 1, except that the reaction temperature in step (1) is 75° C., and the bis (trichloromethyl) carbonate is 25.74 g (0.087 mol) to obtain 116.81 g of dimerized tall oil. acid chloride;
[0037] (2) method is with embodiment 1, and the difference is that the sodium azide in step (2) is 52.03g (0.41mol);
[0038] (3) The method is the same as that in Example 1, except that the reaction temperature in step (3) is 67° C. to obtain 84.52 g of a brown-red transparent oily liquid with a total yield of 76.01%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com