Selective separation method of diameter of single-wall carbon nano tube
A single-walled carbon nanotube, selective technology, applied in the field of carbon nanotube separation, can solve problems such as incompleteness, single-walled carbon nanotube winding, and insufficient environmental protection, and achieve a strong practical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
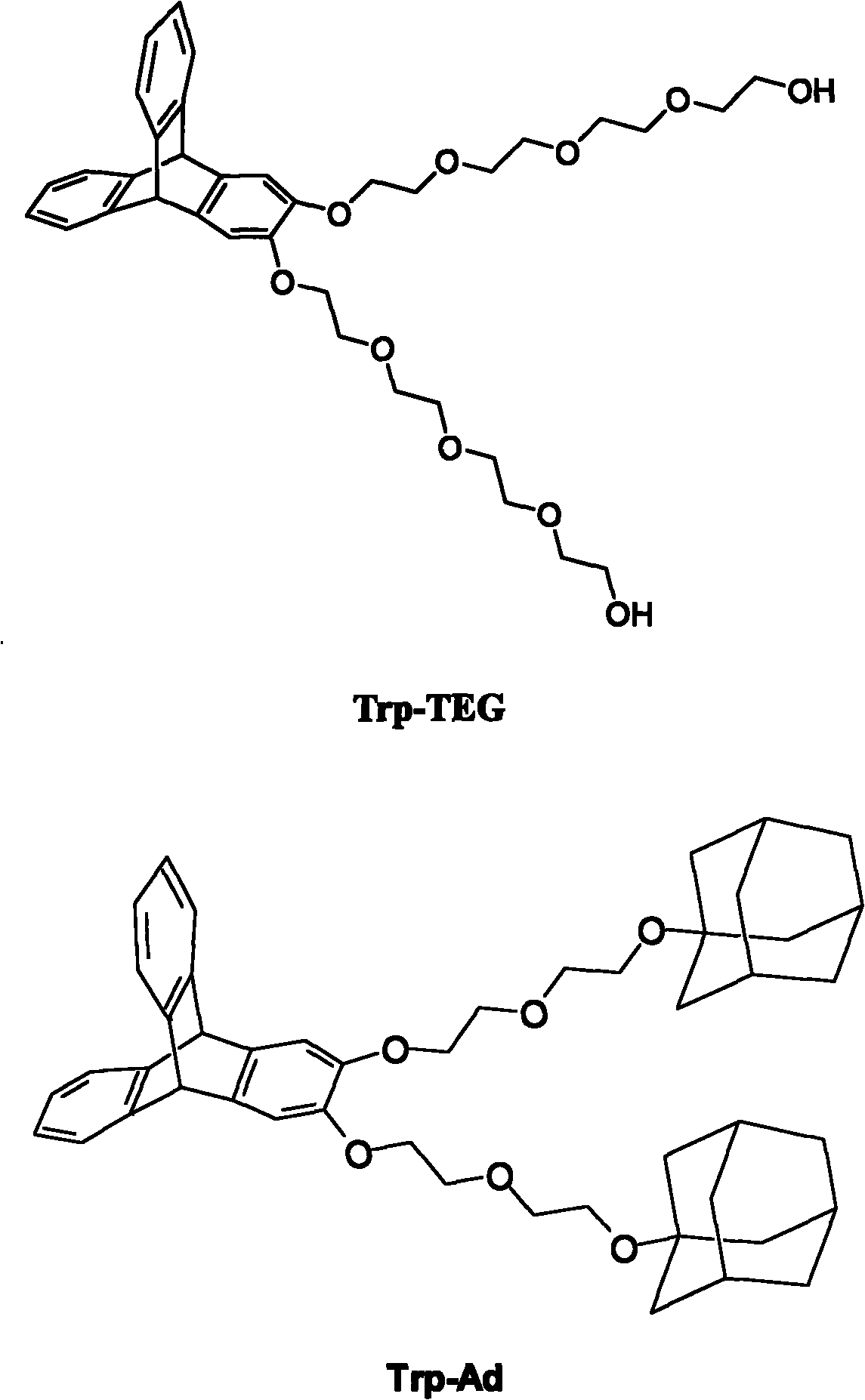
[0038] Triptycene derivative Trp-TEG is composed of dihydroxytriacene Trp(OH) 2 And the synthesis of unilaterally substituted tetraethylene glycol, in which dihydroxytriacene Trp(OH) 2 and unilaterally substituted tetraethylene glycol obtained by methods known in the art, such as Peng, X.-X.; Lu, H.-Y.; Han, T.; Chen C.-F; Synthesis of aNovel Triptycene- Based Molecular Tweezer and Its Complexation with Paraquat Derivatives. Org. Lett. 2007, 9(5), 895-898. Synthetic steps include: under nitrogen protection, 0.57g Trp(OH) 2 , 1.53g unilaterally substituted tetraethylene glycol and 0.69g potassium carbonate mixture were refluxed in acetonitrile for 24 hours. The reaction was cooled to room temperature, then filtered. The resulting solution was concentrated, and then separated by column chromatography, wherein dichloromethane and methanol were eluted at a volume ratio of 100:1 to finally obtain 1.01 g of a colorless oily product. Utilize BRUKER DMX 300 solid-liquid dual-purpo...
Embodiment 2
[0042] Triptycene derivative Trp-TEG is composed of dihydroxytriacene Trp(OH) 2 And the synthesis of unilaterally substituted tetraethylene glycol, in which dihydroxytriacene Trp(OH) 2 and unilaterally substituted tetraethylene glycol obtained by methods known in the art, such as Peng, X.-X.; Lu, H.-Y.; Han, T.; Chen C.-F; Synthesis of aNovel Triptycene- Based Molecular Tweezer and Its Complexation with Paraquat Derivatives. Org. Lett. 2007, 9(5), 895-898. Synthetic steps include: under nitrogen protection, 0.57g Trp(OH) 2 , 1.53g unilaterally substituted tetraethylene glycol and 0.69g potassium carbonate mixture were refluxed in acetonitrile for 24 hours. The reaction was cooled to room temperature, then filtered. The resulting solution was concentrated, and then separated by column chromatography, wherein the elution was dichloromethane and methanol, and their volume ratio was 100:1, and 1.01 g of colorless oily product Trp-TEG was finally obtained. Utilize BRUKER DMX 30...
Embodiment 3
[0046] Triptycene derivative Trp-Ad is composed of dihydroxytriacene Trp(OH) 2 And the synthesis of unilaterally substituted adamantane ethanol, in which dihydroxytriacene Trp(OH) 2 and unilaterally substituted tetraethylene glycol obtained by methods known in the art, such as Peng, X.-X.; Lu, H.-Y.; Han, T.; Chen C.-F; Synthesis of aNovel Triptycene- Based Molecular Tweezer and Its Complexation with Paraquat Derivatives. Org. Lett. 2007, 9(5), 895-898. Synthetic steps include: under nitrogen protection, 0.57g Trp(OH) 2 , a mixture of 1.74g unilaterally substituted adamantane ethanol and 0.69g anhydrous potassium carbonate was refluxed in acetonitrile for 24 hours. The reaction was cooled to room temperature, then filtered. The resulting solution was concentrated, and then separated by column chromatography, wherein the eluent was dichloromethane and methanol in a volume ratio of 30:1, and finally 0.94 g of a colorless oily product was obtained. Utilize BRUKER DMX 300 soli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com