Method for synthesizing permanent violet
A permanent violet and reaction technology, applied in chemical instruments and methods, organic dyes, oxazine dyes, etc., can solve the problems of difficult control of reaction temperature, low utilization rate of raw materials, and many by-products, so as to reduce raw material consumption and reaction time Effect of shortening and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
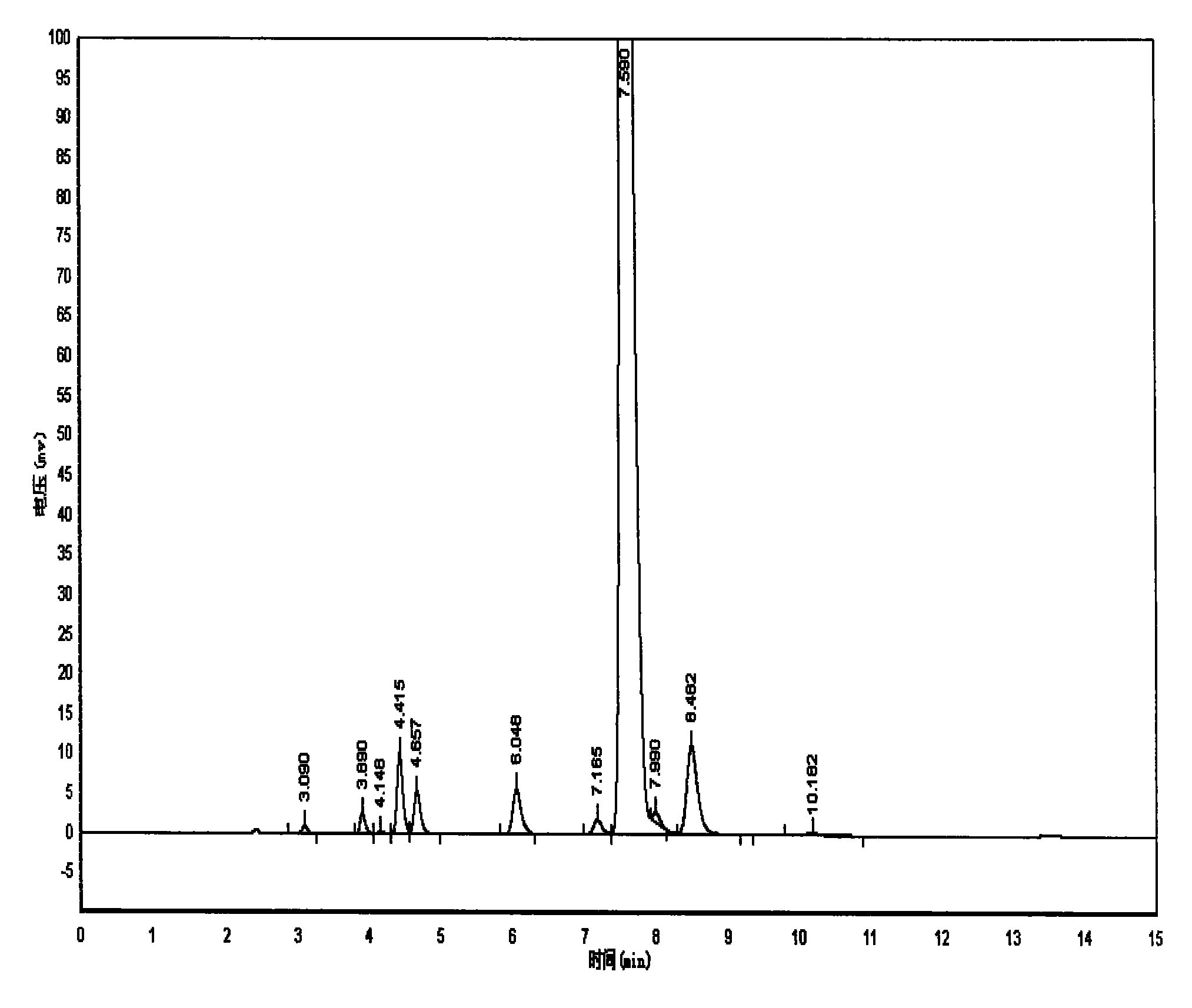
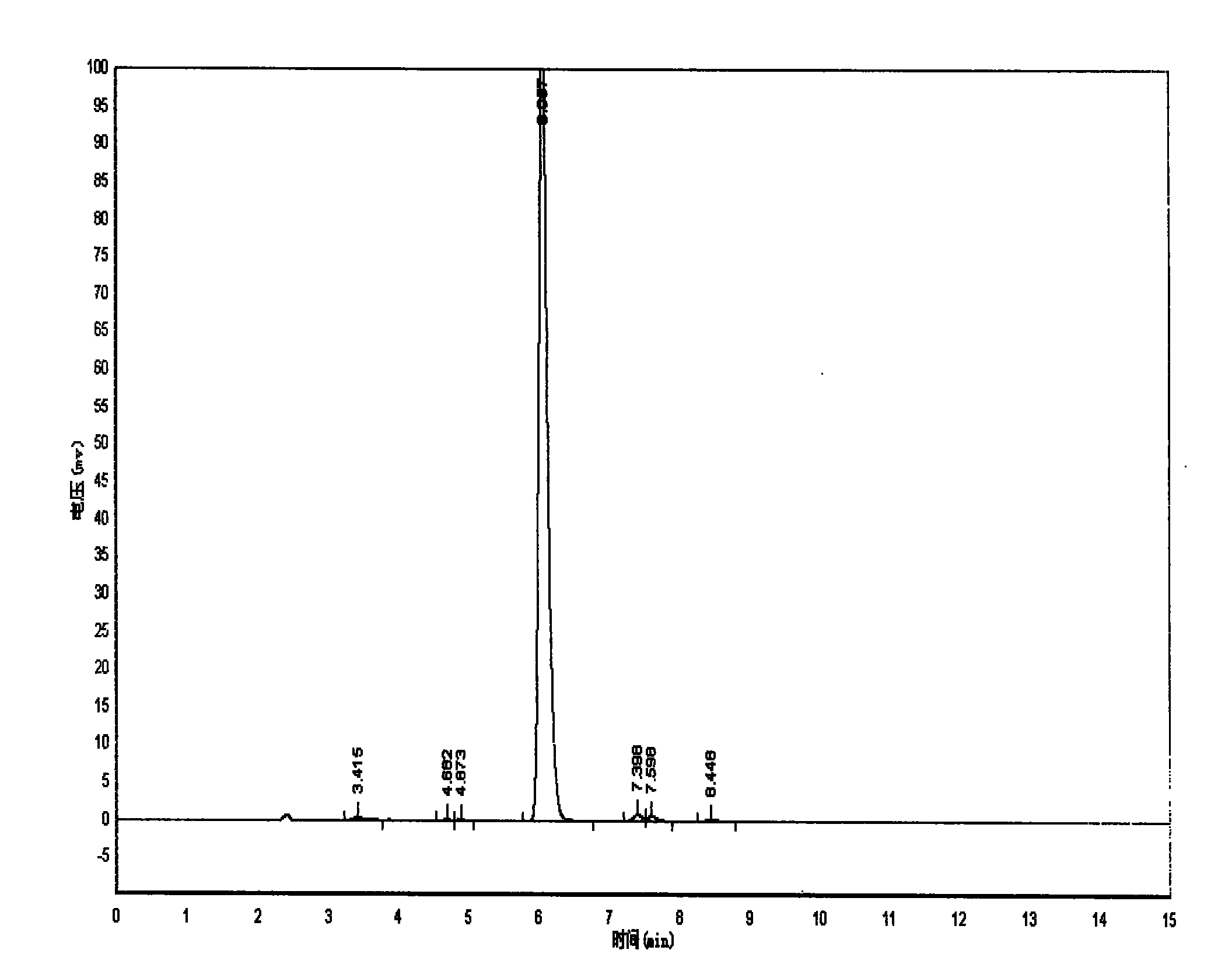
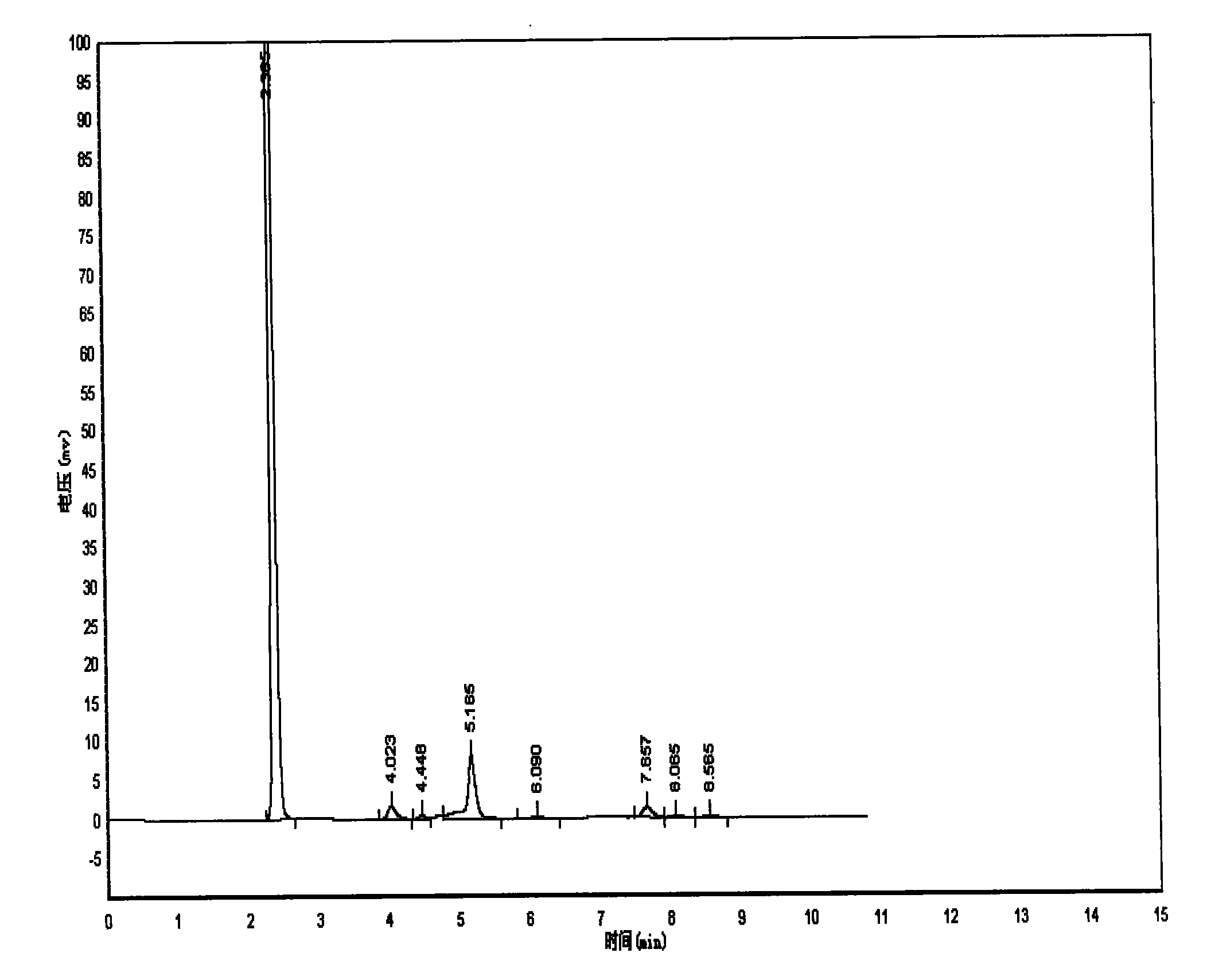
Image
Examples
Embodiment Construction
[0020] The method steps of the present invention are as follows:
[0021] 1. Alkylation: first prepare liquid caustic soda, add an appropriate amount of alkylation recovered liquid caustic to a steel reactor, and mix with solid caustic (industrial grade) to prepare liquid caustic with a total alkalinity of 40-43% ; Resynthesize N-ethylcarbazole, take 150Kg of liquid caustic soda prepared above (total alkalinity: 40-43%), add it to a 500L pressure reactor with stirring, thermometer, nitrogen tube and sampling port, and then Add 71Kg chlorobenzene, 60.6Kg (purity ≥96%) carbazole, 0.6Kg catalyst, 40.5Kg bromoethane (control the temperature below 30℃), after the addition, cover the kettle, replace it with nitrogen three times, then open Stir, under stirring, heat up to 65~70℃ in about 1 hour, continue to stir and react at this temperature for 4 hours, take samples and check, the end point is that the carbazole spots disappear; the reaction is over, the reaction solution is transferre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com