Indole carbazole and bisindole maleimide alkaloid, preparation method and application thereof
A derivative, diindole technology, applied in the application field of tumor treatment, can solve the problems of poor curative effect, large toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
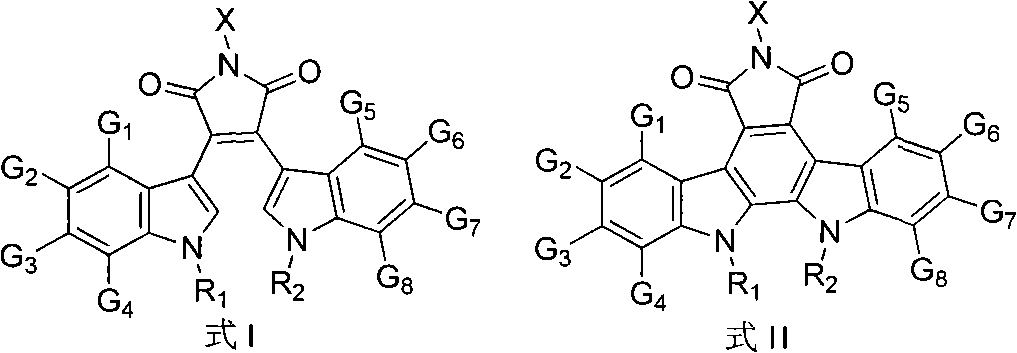
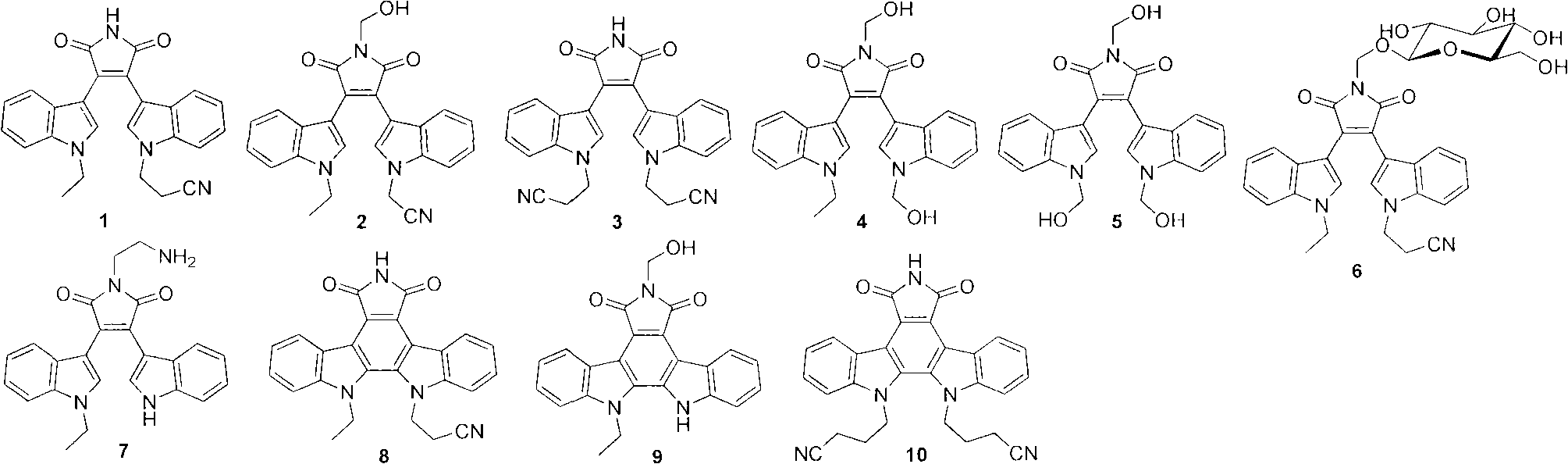
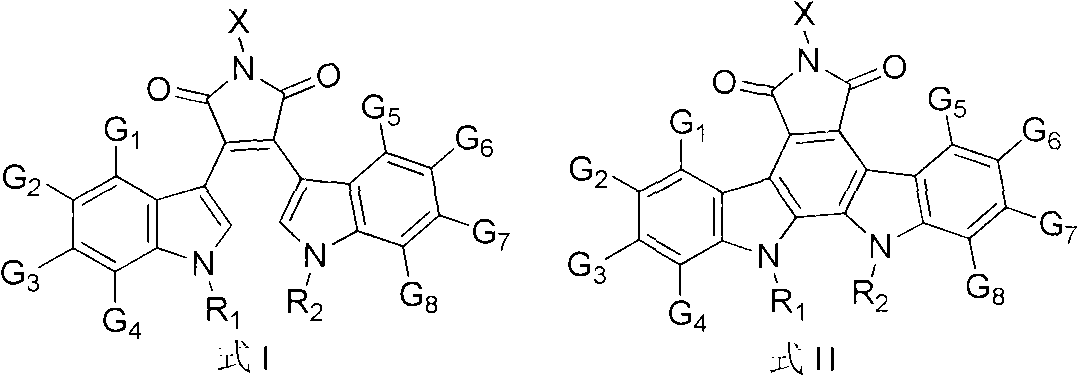
[0017] [Example 1] Preparation of Compounds 1-10
[0018] Preparation of Compound 1
[0019] i) Preparation of 2-(1-ethyl-1H-indol-3-yl) acetic acid (1a)
[0020]Under the protection of argon, in a 250mL three-necked flask, add sodium hydride (4g, 100mmol, 60% dispersed in paraffin), add 80mL tetrahydrofuran and stir to suspend at 0°C, add 30mL tetrahydrofuran dissolved indole-3-acetic acid (3.5 g, 20mmol), after stirring for half an hour, add 30mL tetrahydrofuran dissolved ethyl iodide (5mL, 60mmol) dropwise, slowly rise to room temperature, after reacting overnight, drop down to 0°C, add dropwise 10 drops of methanol, and then add appropriate amount of water To obtain a bright yellow solution, extract with ethyl acetate, add concentrated hydrochloric acid to the aqueous layer and extract again, combine the organic layers, and wash with anhydrous Na 2 SO 4 Dry, evaporate to dryness in vacuo, and separate through silica gel column (petroleum ether: ethyl acetate 8:1) to obt...
Embodiment 2
[0083] [Example 2] Test of antitumor activity
[0084] 1 Cytotoxic activity
[0085] 1.1 Test method
[0086] Preparation of the test sample solution: the test samples are the monomer compounds 1-10 synthesized in the above-mentioned Example 1. Accurately weigh an appropriate amount of sample and prepare a solution with the required concentration with DMSO for activity testing.
[0087] Cell lines and subculture of cells: A549, BEL-7402 and HL-60 cell lines were used for activity testing. All kinds of cells were subcultured in RPMI-1640 medium containing 10% FBS in an incubator with 5% carbon dioxide at 37°C.
[0088] Cell Proliferation Inhibitory Activity Test Method
[0089] The invention adopts the MTT method to test and evaluate the inhibitory activity of the tested sample on the proliferation of HL-60 cancer cells. The dehydrogenase in the mitochondria of living cells can metabolize and reduce the yellow 3-(4,5-dimethylthiazole)-2,5-diphenyltetrazolium bromide to blu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com