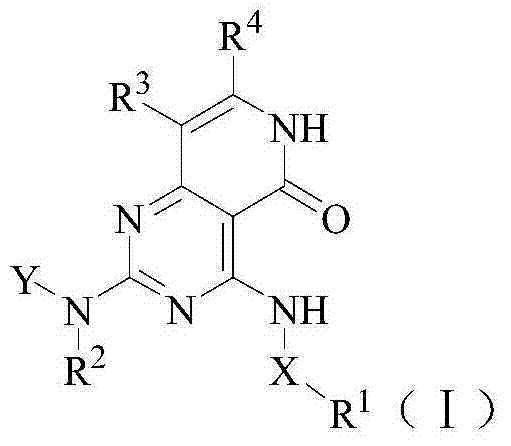
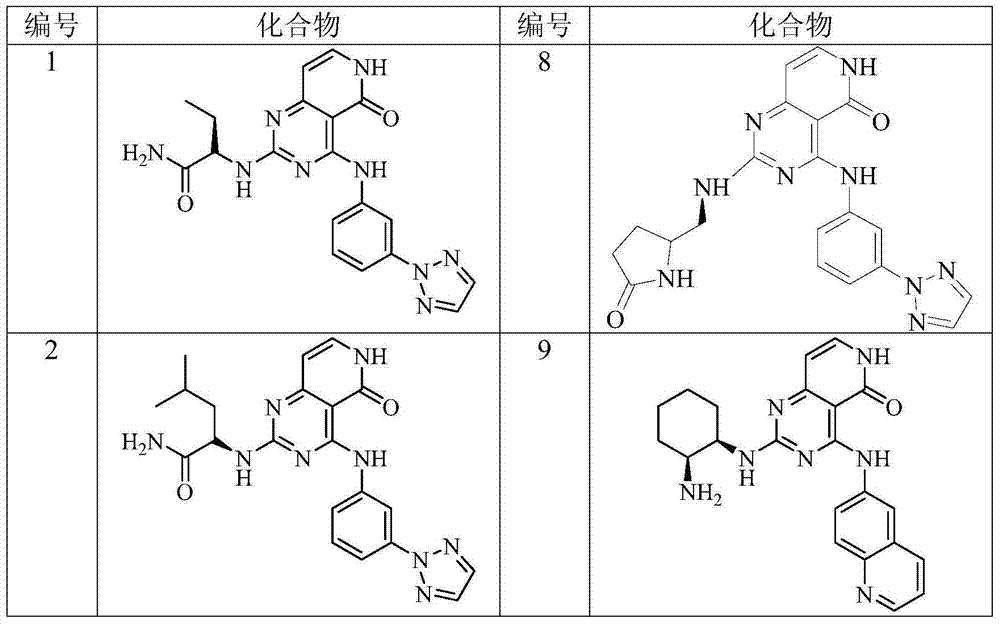
Benzopyrimidine-containing SYK inhibitor
A monocyclic and cycloalkyl technology, applied in medical preparations containing active ingredients, anti-inflammatory agents, organic chemistry, etc., can solve problems such as high price, difficult promotion, and large side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179] Example 1 4-[3-(2H-1,2,3-triazol-2-yl)anilino]-2-((cis)-2-aminocyclohexylamino)pyrido[4, 3-d] Preparation of pyrimidin-5(6H)-one (compound 4)
[0180] (1) ethyl 4-[3-(2H-1,2,3-triazol-2-yl)anilino]-6-methyl-2-(methylmercapto)pyrimidine-5-carboxylate
[0181]
[0182] Ethyl 4-chloro-6-methyl-2-(methylmercapto)pyrimidine-5-carboxylate (2.5g, 10.13mmol), 3-(2H-1,2,3-triazol-2-yl ) aniline (1.78g, 11.14mmol), and sodium acetate trihydrate (3.03g, 22.3mmol) were dissolved in 40mL of ethanol, heated at 80°C for overnight reaction, the solvent was evaporated to dryness, and the residue was dissolved in ethyl acetate, washed with water, and dried After concentration, it was separated by silica gel column (dichloromethane:methanol=100:1) to obtain 2.25g of product, the yield was 60%.
[0183] (2) 4-[3-(2H-1,2,3-triazol-2-yl)anilino]-6-methyl-2-(methylmercapto)pyrimidine-5-carboxylic acid
[0184]
[0185] Ethyl 4-[3-(2H-1,2,3-triazol-2-yl)anilino]-6-methyl-2-(methylmer...
Embodiment 2
[0200] Example 2 (S)-2-[4-[3-(2H-1,2,3-triazol-2-yl)anilino]pyrido[4,3-d]pyrimidine-5(6H) Preparation of -keto-2-ylamino]butyramide (Compound 1)
[0201]
[0202] Operation is the same as in Example 1 (6), and the productive rate is 9.7%.
[0203] Mass Spectrum (m / e): 405.9 (M+1)
[0204] 1 H-NMR (400MHz, d 6 -DMSO, δ ppm ):0.94(m, 3H), 1.85(m, 2H), 4.43(m, 1H), 6.13(m, 1H), 7.05(s, 1H), 7.18(m, 1H), 7.4(m, 2H) , 7.54(m, 1H), 7.75(m, 1H), 7.84~8.06(m, 1H), 8.14(m, 2H), 8.14~8.54(m, 1H), 11.4(m, 1H), 12.2(m , 1H).
Embodiment 3
[0205] Example 3 (S)-2-[4-[3-(2H-1,2,3-triazol-2-yl)anilino]pyrido[4,3-d]pyrimidine-5(6H) Preparation of -keto-2-ylamino]-4-methylpentanamide (compound 2)
[0206]
[0207] Operation is the same as in Example 1 (6), and the productive rate is 5.2%.
[0208] Mass Spectrum (m / e): 434.3 (M+1)
[0209] 1 H-NMR (400MHz, CD 3 OD, δ ppm ):0.88~1.0(m, 6H), 1.29(m, 1H), 1.32(m, 1H), 2.12(m, 1H), 4.60(m, 1H), 6.3(m, 1H), 7.37(m, 1H), 7.51(m, 2H), 7.81(s, 1H), 7.99(m, 2H), 9.04(m, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com