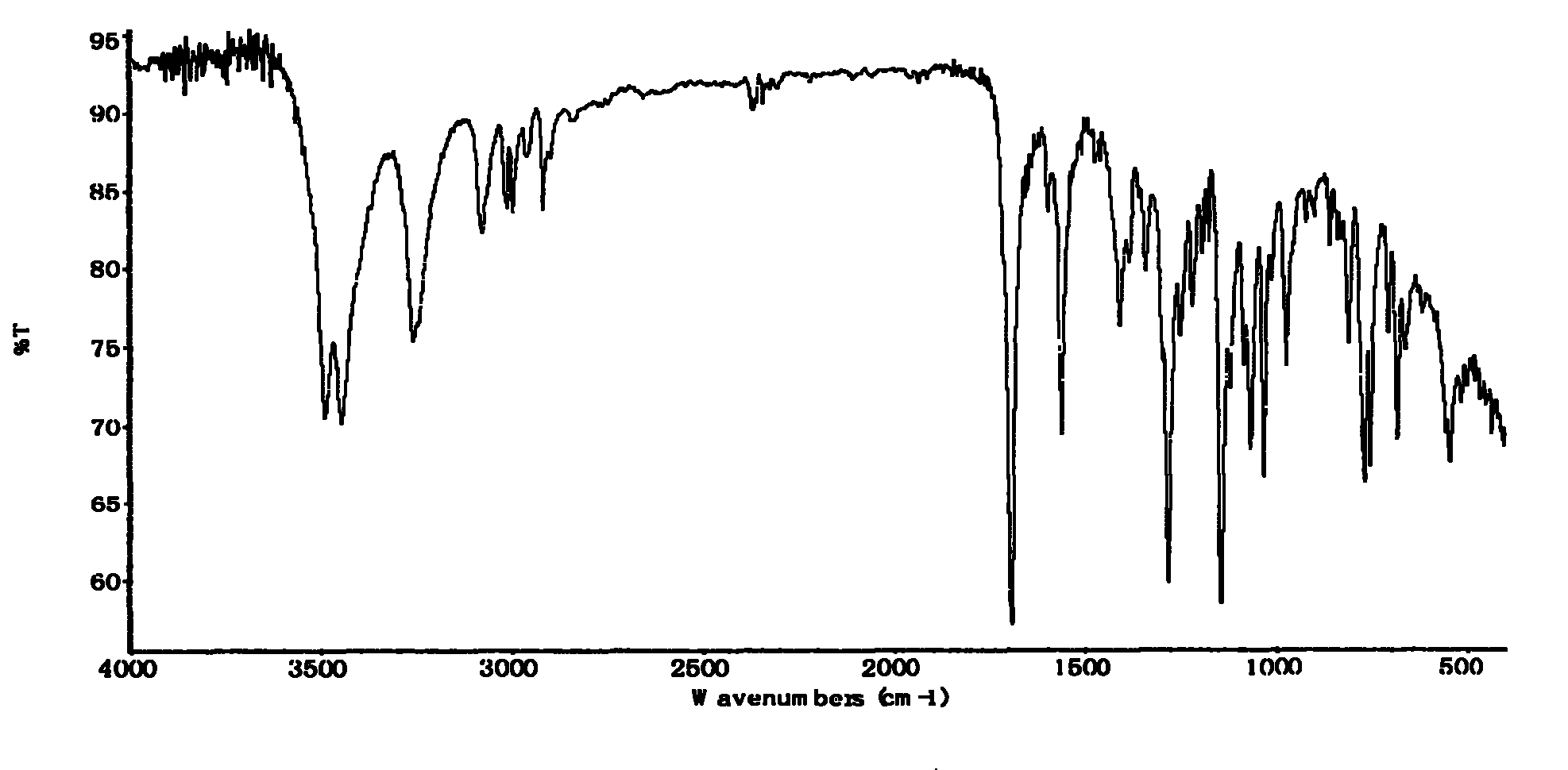
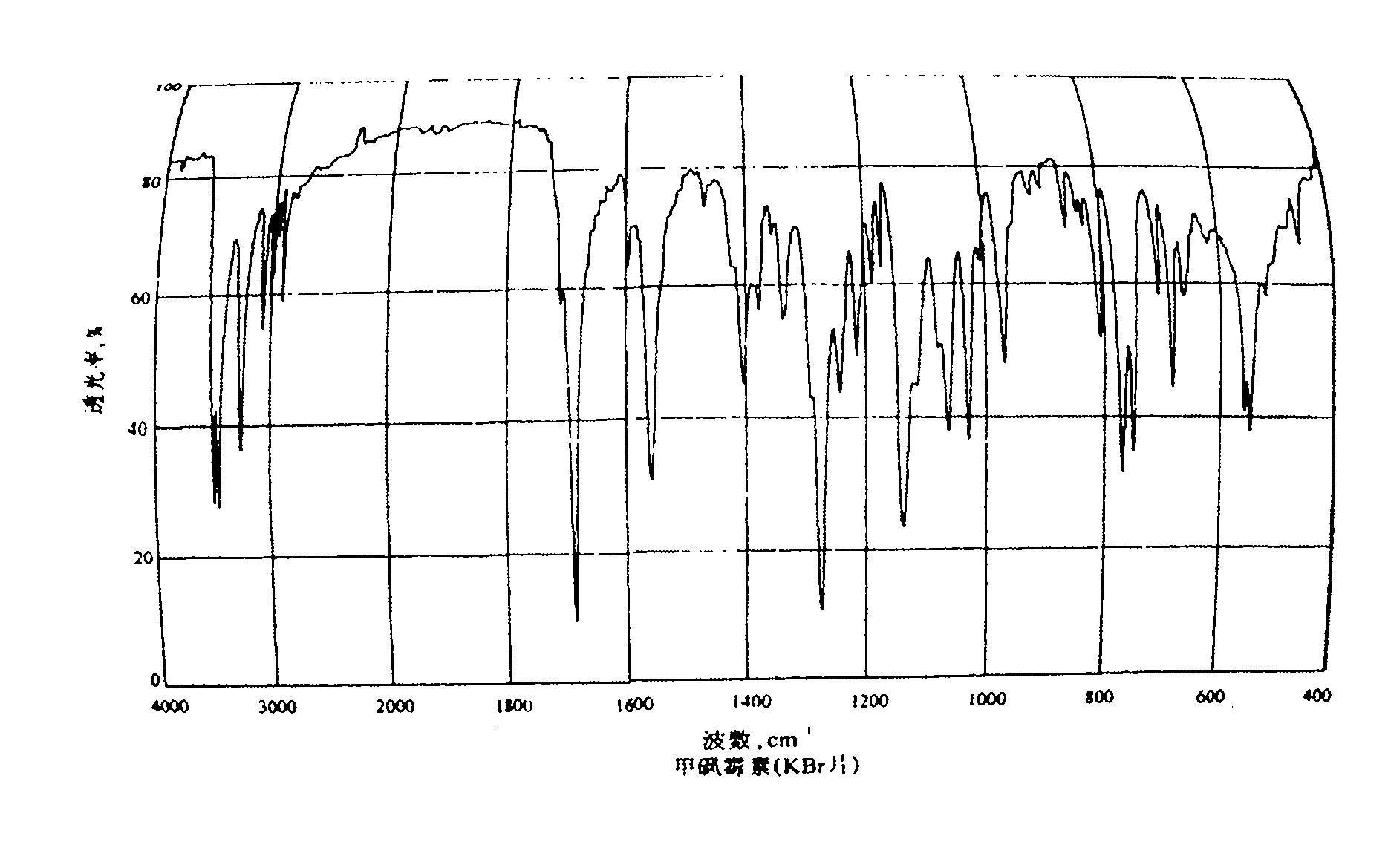
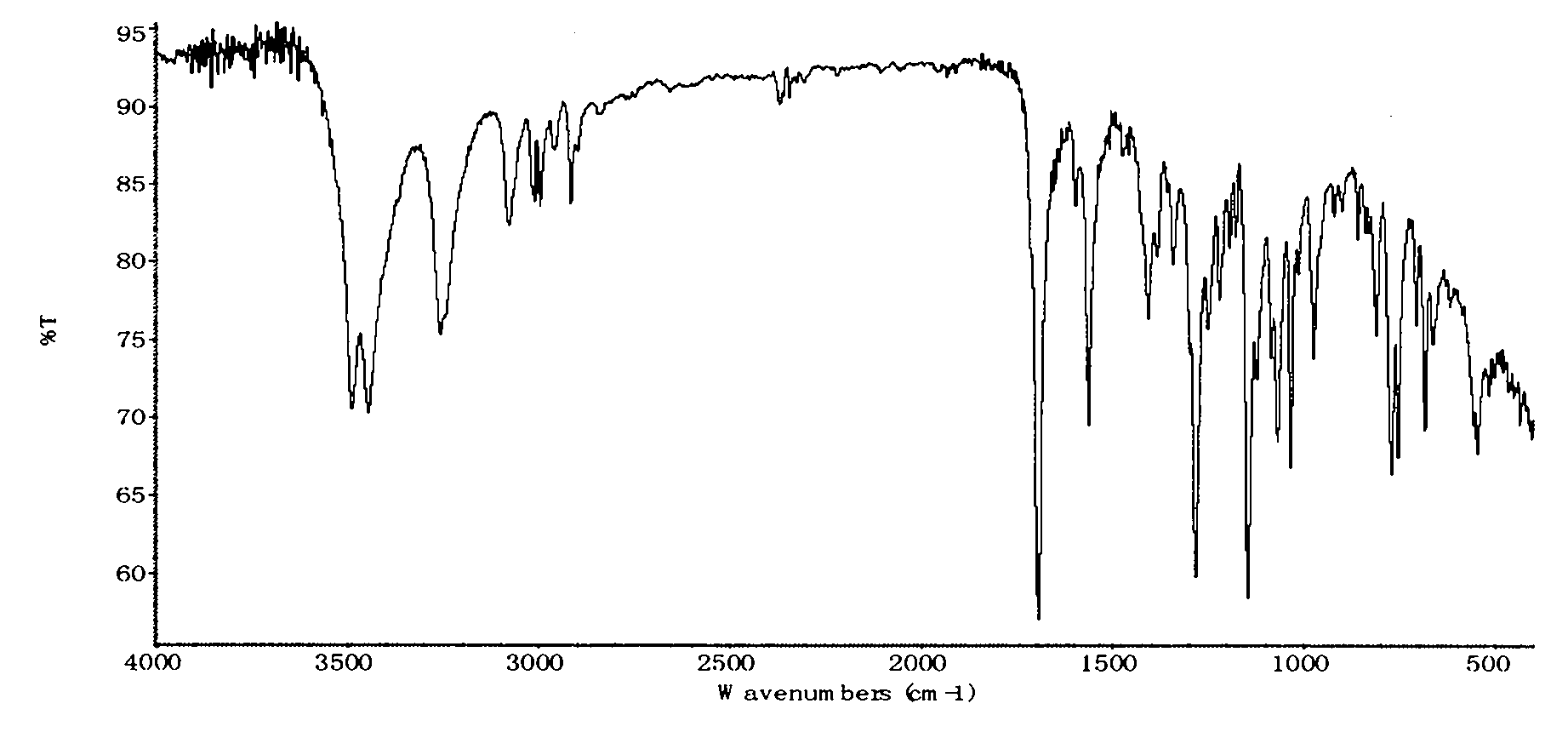
Preparation method of thiamphenicol
A technology of thiamphenicol and methylsulfonyl phenylserine ethyl ester, which is applied in the field of antibiotic preparation, can solve the problems of long process route, long reaction time, many three wastes, etc., and achieves shortened process steps, short reaction time, and reduced waste water. Effect
Active Publication Date: 2010-08-25
JIANGSU HANSYN PHARMA
View PDF3 Cites 5 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
US: 3927054 uses p-thiamphenicol benzaldehyde to produce thiamphenicol through condensation, esterification, resolution, reductive acylation, acidification, etc., which has the disadvantages of long process route, low yield, many wastes, and high production cost; CN101200441A Using D-threo-2-(dichloromethyl)-4,5-dihydro-5-[p-(thymphenyl)phenyl]-4-oxazolemethanol (III) to prepare thiamphenicol element, but the process of purifying raw materials is complex and wastes a large amount of isomer D--threo-2-(dichloromethyl)-4,5-dihydro-a-[p-(thymphenyl)phenyl] -4-oxazole methanol (IV), resulting in low yield, high cost, long reaction time and other shortcomings
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Login to View More
Abstract
The invention relates to a preparation method of antibiotic, in particular to a preparation method of thiamphenicol. The preparation method comprises the following steps: by weight, dissolving 100 parts of D-4-Methylsulfonylphenyl serine ethyl ester in 500-700 parts of methanol solvent, adding 24-27 parts of KBH4 to react at 30-60 DEG C for 4-8 hours, recycling 300-400 parts of methanol, neutralizing reaction solution with acid to adjust the pH value of the solution to 6-10, adding 42-45 parts of dichloroacetonitrile, performing a cyclization reaction for 4-6 hours, reducing pressure to recycle all of the solution, adding water to perform solid-liquid separation and obtain a solid mixture, adding 600-800 parts of water in the solid mixture, heating to 85-90 DEG C, keeping temperature at 85-90 DEG C for 30 minutes, and discoloring to obtain thiamphenicol. The invention is characterized by fewer steps, simple operation, short reaction time, low cost and the like.
Description
technical field The invention relates to a preparation method of antibiotics, in particular to a preparation method of thiamphenicol. Background technique Thiamphenicol is an antibiotic, mainly used for various infectious diseases caused by sensitive bacteria, and is generally produced by synthetic methods. US: 3927054 uses p-thiamphenicol benzaldehyde to produce thiamphenicol through condensation, esterification, resolution, reductive acylation, acidification, etc., which has the disadvantages of long process route, low yield, many wastes, and high production cost; CN101200441A Using D-threo-2-(dichloromethyl)-4,5-dihydro-5-[p-(thymphenyl)phenyl]-4-oxazolemethanol (III) to prepare thiamphenicol element, but the process of purifying raw materials is complex and wastes a large amount of isomer D--threo-2-(dichloromethyl)-4,5-dihydro-a-[p-(thymphenyl)phenyl] -4-oxazole methanol (IV), resulting in low yield, high cost, long reaction time and other shortcomings. Contents of ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07C317/32C07C315/04
Inventor 周留扣唐忠松张汉兴
Owner JIANGSU HANSYN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com