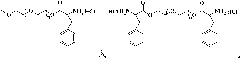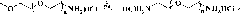Polymer microgel and preparation method thereof
A polymer and microgel technology, applied in the field of polymer synthesis, can solve problems such as poor biocompatibility and non-biodegradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
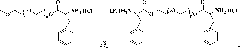
[0061] The present invention also provides a preparation method of polymer microgel, comprising: a) preparing polyethylene glycol monomethyl ether-poly(L-glutamic acid) block copolymer or polyethylene glycol-poly(L -glutamic acid) block copolymer;
[0062] b) Introducing 3-phenyl-2-propenyl or 2-methacryloyloxyethyl into the block copolymer segment prepared in step a) to obtain polyethylene glycol monomethyl ether-b-poly(L -glutamic acid)-co-poly(γ-3-phenyl-2-propenyl-L-glutamate), polyethylene glycol monomethyl ether-b-poly(L-glutamic acid)-co -poly(γ-2-methacryloyloxyethyl-L-glutamate), poly(L-glutamic acid)-co-poly(γ-3-phenyl-2-propenyl-L- glutamate)-b-polyethylene glycol-b-poly(L-glutamate)-co-poly(γ-3-phenyl-2-propenyl-L-glutamate) or poly( γ-2-methacryloyloxyethyl-L-glutamate)-b-polyethylene glycol-b-poly(L-glutamate)-co-poly(γ-2-methacryloyl Oxyethyl-L-glutamate);
[0063] c) dissolving the block copolymer prepared in step b), and then undergoing a crosslinking reac...
Embodiment 1
[0098] Embodiment 1: the preparation (coupling method) of the phenylalanine polyethylene glycol monomethyl ether ester of different number-average molecular weights
[0099]Take by weighing 10g number average molecular weight respectively and be 1000 (0.01mol), 2000 (0.005mol), 5000 (0.002mol), 10000 (0.001mol), the polyethylene glycol monomethyl ether of 20000 (0.0005mol), put into 5 respectively In a dry reaction flask with branched mouth, after adding 100mL of toluene to azeotropically remove water, add 26.53g (0.1mol), 13.265g (0.05mol), 5.306g (0.02mol), 2.653 (0.01mol), 1.3265 g (0.005mol) of phenylalanine with tert-butoxycarbonyl-protected amino groups were dissolved in 365.3mL, 232.65mL, 153.06mL, 126.53mL, and 113.265mL of anhydrous chloroform, cooled to 0°C, and 10.317 g, 5.158g, 2.063g, 1.032g, 0.516g N,N'-cyclohexylcarbodiimide and 1.222g, 0.611g, 0.244g, 0.122g, 0.061g 1,4-dimethylaminopyridine. After all the solids were dissolved, a clear solution was obtained, ...
Embodiment 2
[0104] Embodiment 2: the preparation (coupling method) of the phenylalanine polyethylene glycol ester of different number-average molecular weights
[0105] Prepare phenylalanine polyethylene glycol ester according to the method of embodiment 1, different from embodiment 1, respectively get 5g number-average molecular weight polyethylene glycol identical with embodiment 1, take by weighing 1g number-average molecular weight respectively and be 1494( 6.69×10 -4 mol), 2494 (4.01×10 -4 mol), 5494 (1.82×10 -4 mol), 10494 (9.53×10 -5 mol), 20494 (4.88×10 -5 mol) tert-butoxycarbonyl-protected amino-phenylalanine polyethylene glycol ester, other steps and data are the same. The productive rate of each step obtained product is shown in Table 2.
[0106] The number-average molecular weight and reaction yield of each step gained product of table two
[0107] experiment number
[0108] In the above table, Mn 1 Be the number-average molecular weight of the phenylalanine p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com