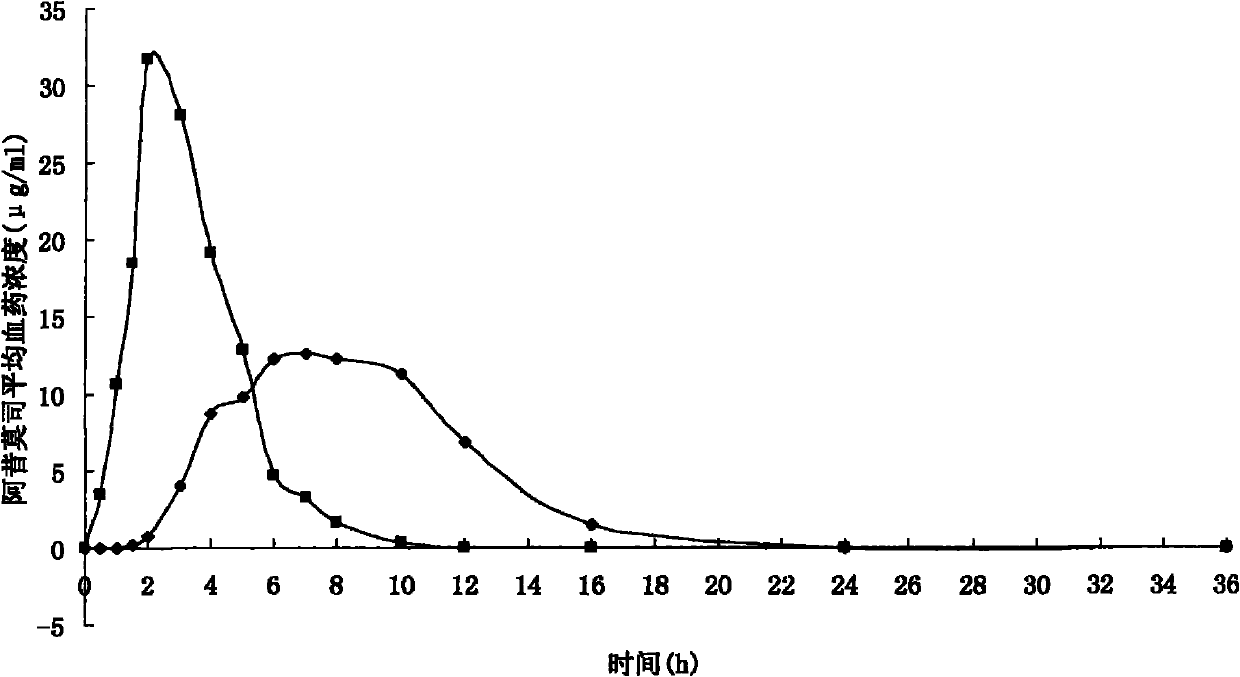
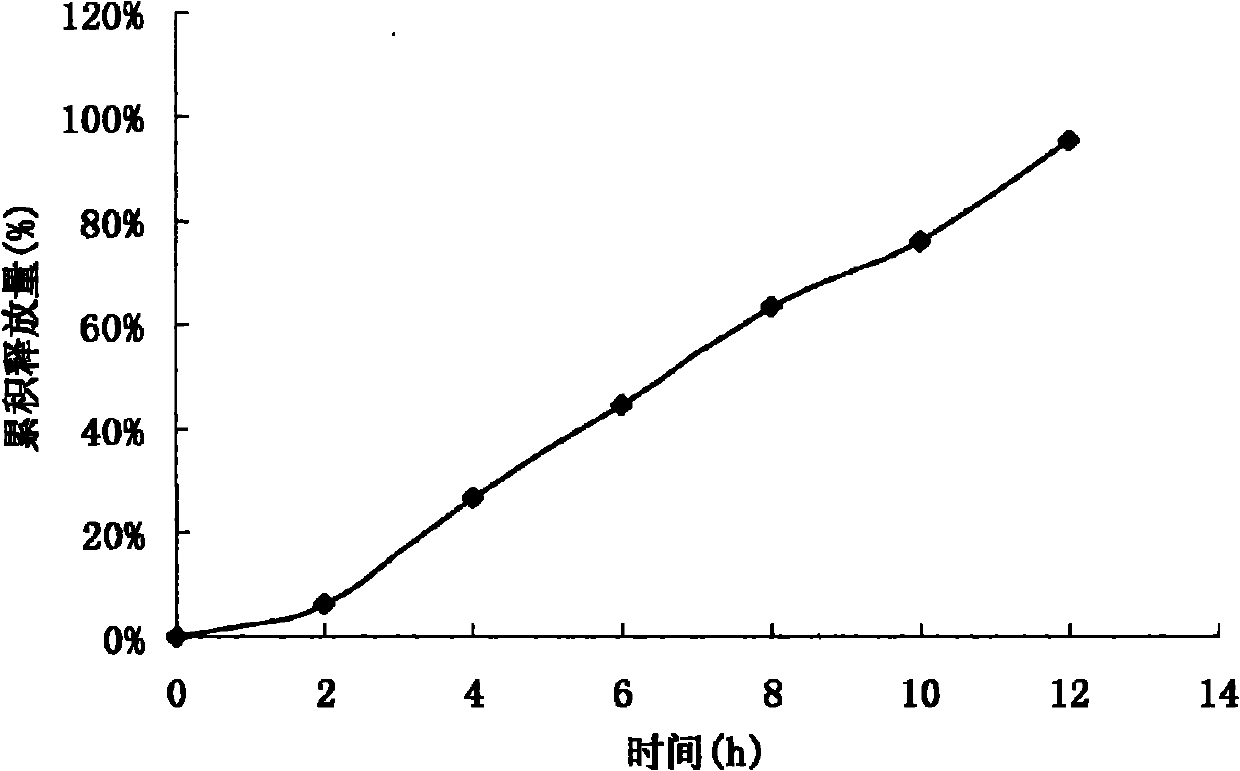
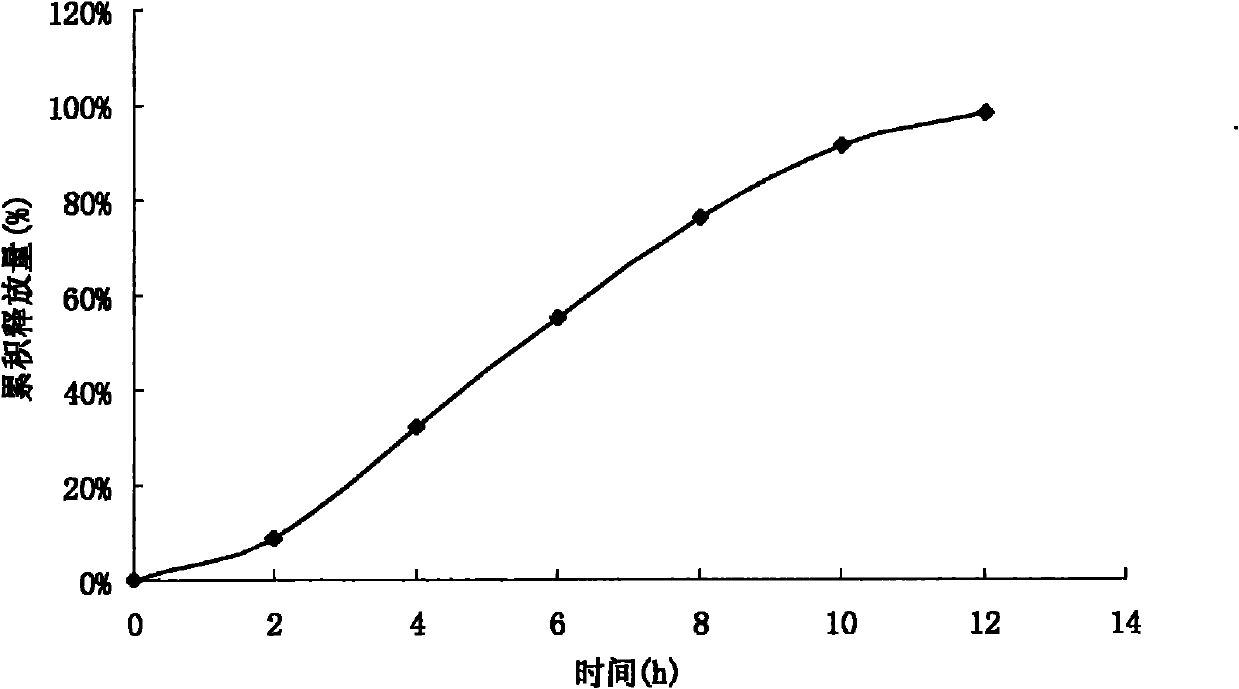
Acipimox push-pull osmotic pump controlled release tablet and preparation method thereof
A technology of osmotic pump controlled release and acyclolimus, which is applied in the field of acyclomus double-layer osmotic pump controlled release tablet and its preparation, and achieves the effect of overcoming the frequent taking and maintaining blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Tablet prescription:
[0026] Drug-containing layer
[0027] Acipimox 250mg
[0028] Polyoxyethylene 200mg
[0029] Mannitol 20mg
[0031] Anhydrous ethanol amount
[0032] booster layer
[0033] Sodium Alginate 90mg
[0034] Sucrose 15mg
[0035] Semi-permeable coating film prescription:
[0036] Cellulose acetate 18g
[0037] Macrogol 4000 4g
[0038] Solvent prescription for dissolving film coating material:
[0039] Acetone 1000mL
[0040] Distilled water 20mL
[0041] Preparation Process:
[0042] Pass the prescribed amount of medicine, polyoxyethylene with a molecular weight of 200,000, and mannitol through an 80-mesh sieve, mix evenly, use absolute ethanol as a binder to make a soft material, and granulate with a 20-mesh sieve. After the wet granules are dried, sieve through a 18-mesh sieve Granules, dry granules and lubricants are...
Embodiment 2
[0044] Tablet prescription:
[0045] Drug-containing layer
[0046] Acipimox 250mg
[0047] Gum Arabic 300mg
[0050] 95% ethanol appropriate amount
[0051] booster layer
[0052] Polyoxyethylene PEO WSR301 100mg
[0053] Lactose 10mg
[0054] Semi-permeable coating film prescription:
[0055] Ethyl cellulose 20g
[0056] Macrogol 6000 4g
[0057] Solvent prescription for dissolving film coating material:
[0058] Acetone 500mL
[0059]Distilled water 30mL
[0060] Preparation Process:
[0061] Pass the prescribed amount of medicine, gum arabic, and sodium chloride through an 80-mesh sieve, mix evenly, use 95% ethanol as a binder to make a soft material, granulate with a 20-mesh sieve, and after the wet granules are dried, granulate with an 18-mesh sieve and dry The drug layer is obtained after the granules and lubricant a...
Embodiment 3
[0063] Tablet prescription:
[0064] Drug-containing layer
[0065] Acipimox 250mg
[0066] Polyoxyethylene 150mg
[0067] Lactose 10mg
[0068] Micronized silica gel 3mg
[0069] Anhydrous ethanol amount
[0070] booster layer
[0071] Polyoxyethylene 130mg
[0073] Semi-permeable coating film prescription:
[0074] Cellulose acetate 12g
[0075] Acrylic 8
[0076] Macrogol 1500 5g
[0077] Solvent prescription for dissolving film coating material:
[0078] Acetone 1000mL
[0079] Distilled water 30mL
[0080] Preparation Process:
[0081] Pass the prescribed amount of medicine, polyoxyethylene with a molecular weight of 500,000, and lactose through a 80-mesh sieve, mix evenly, use absolute ethanol as a binder to make a soft material, granulate with a 20-mesh sieve, and dry the wet granules, then sieve through a 18-mesh sieve Granules, d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com