Stably isoosmotic desmoteplase alpha1 or mutant preparation thereof
An aminoplase and mutant technology, applied in the field of biomedicine, can solve the problems of loss of activity, difficult recovery of activity, change of tertiary structure, etc., and achieve the effects of improving stability, easy acceptance, and easy storage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
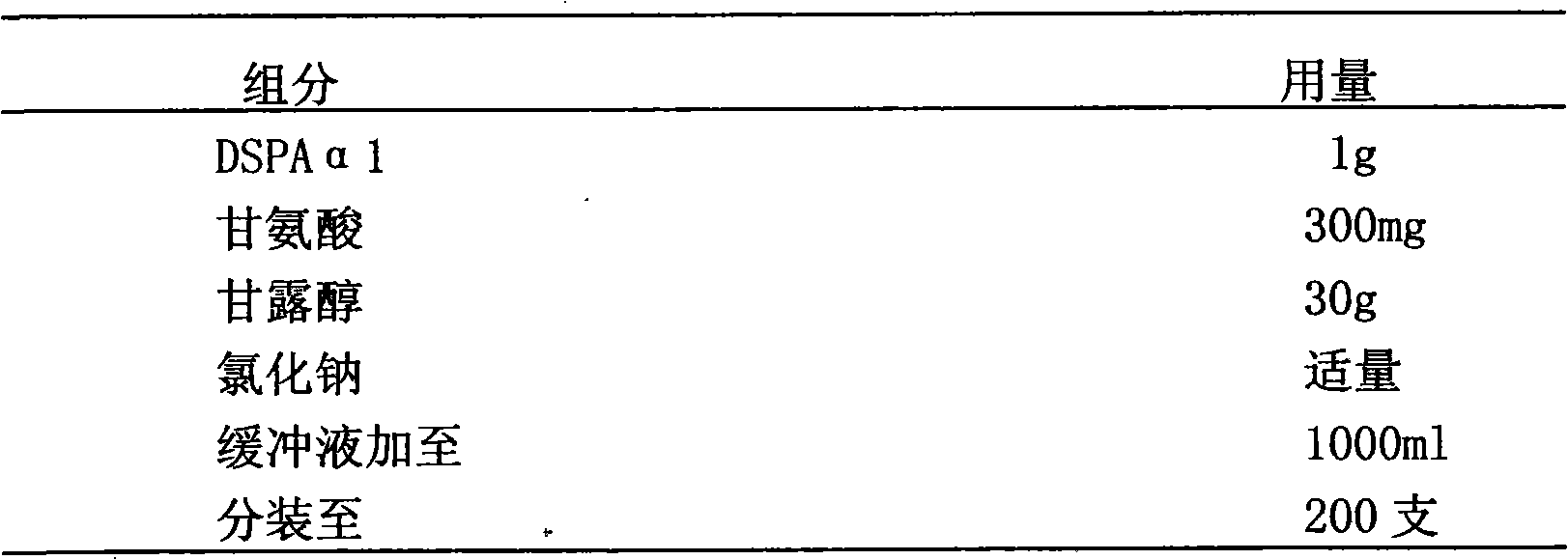
[0036] Example 1: Preparation of a stable isotonic DSPAα1 lyophilized preparation with 10 mM phosphate buffer at pH 5.8 as a buffer system
[0037] Detailed prescription
[0038]
[0039] Preparation:
[0040] Prepare the prescribed amount of 10mM phosphate buffer solution with pH 5.8, accurately weigh the excipients in the prescribed amount, add an appropriate amount of phosphate buffer solution to dissolve, adjust the pH value to 5.5-6.5, and set the volume to the total volume of the solution Difference from DSPAα1 stock solution. Add the prescribed amount of DSPAα1 stock solution into it, shake gently to mix, sterilize and filter with a microporous membrane with a pore size of 0.22 μm, subpackage, freeze-dry, stopper, and press the cap to obtain the product.
Embodiment 2
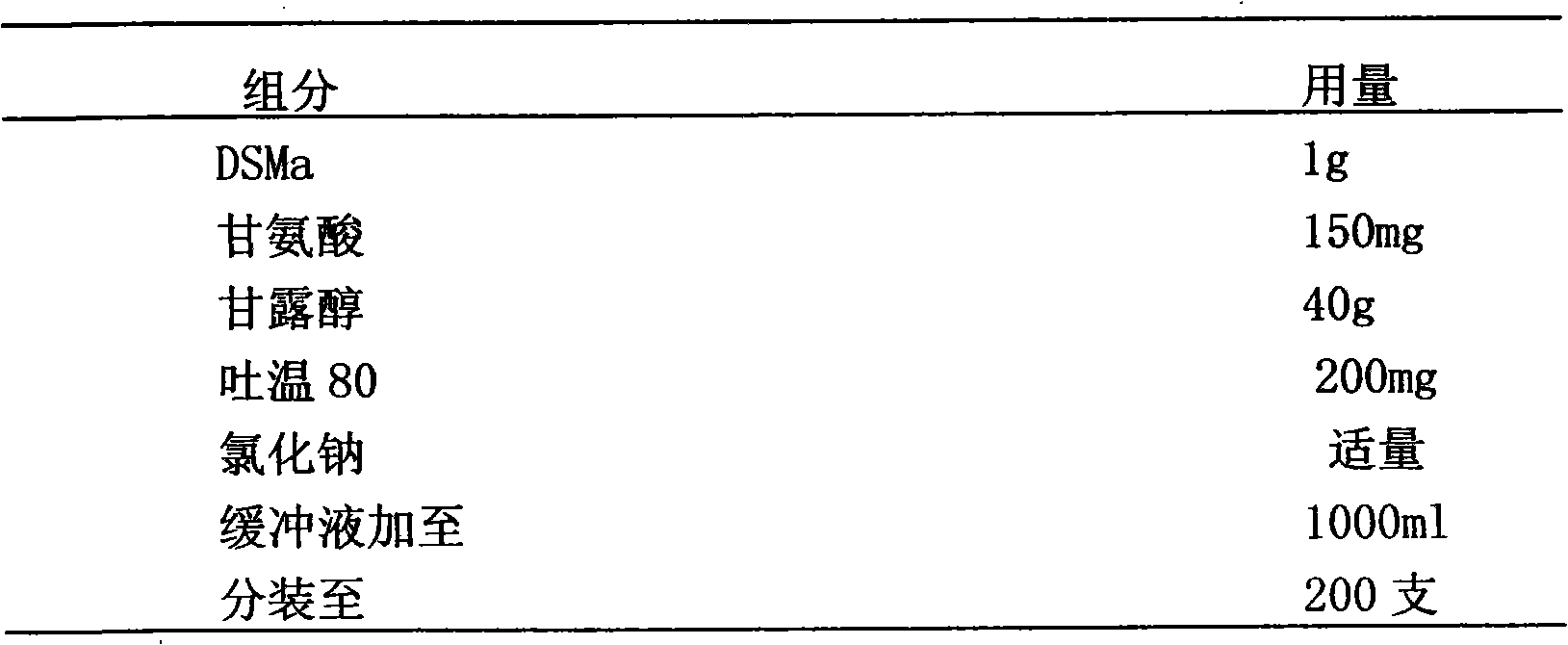
[0041] Embodiment 2: 20mM phosphate buffer with pH 6.0 is buffer system and prepares stable isotonic DSPA α1 mutant DSMa lyophilized preparation
[0042] prescription composition
[0043]
[0044] Preparation:
[0045] Prepare the prescribed amount of 20mM phosphate buffer solution with pH 6.0, accurately weigh the excipients in the prescribed amount, add an appropriate amount of phosphate buffer solution to dissolve, adjust the pH value to 5.5-6.5, and set the volume to the total volume of the solution Difference with DSMa stock solution. Add the prescribed amount of DSMa stock solution into it, shake gently to mix, sterilize and filter with a microporous filter membrane with a pore size of 0.22 μm, sub-package, freeze-dry, stopper, and cap, to obtain.
Embodiment 3
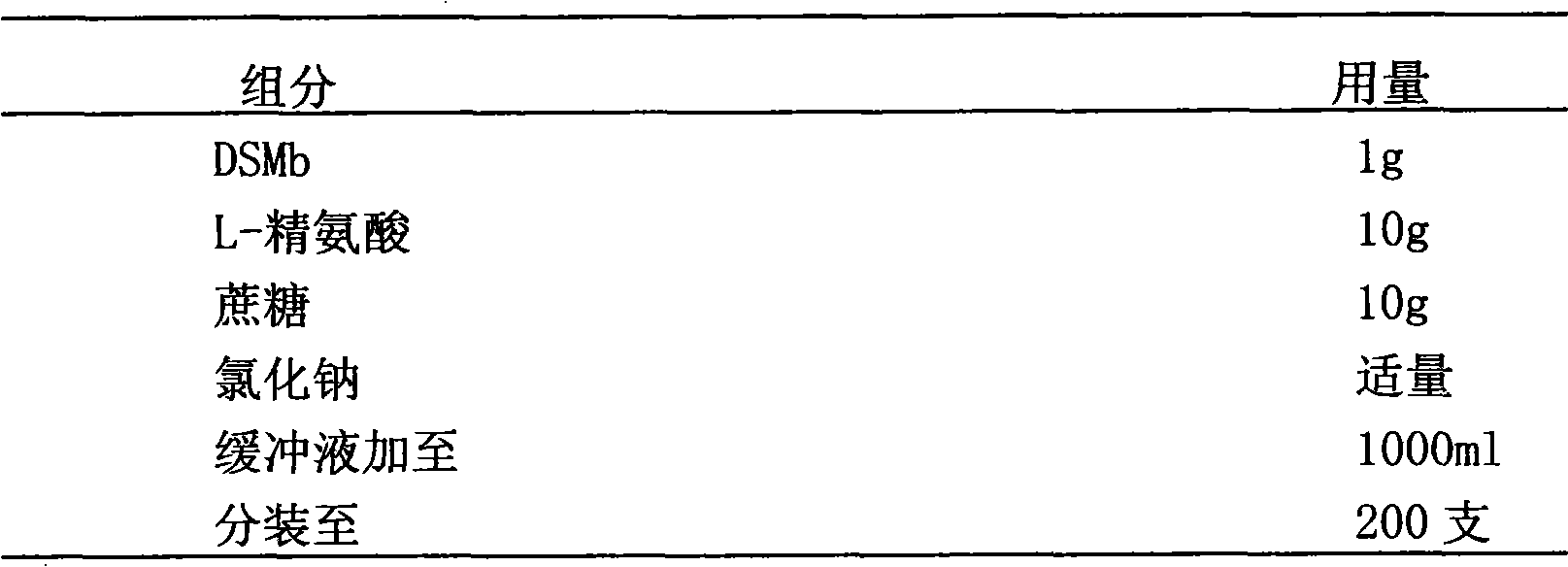
[0046] Example 3: Preparation of stable isotonic DSPA α1 mutant DSMb lyophilized preparation with 0.1M citrate buffer at pH 6.2 as buffer system
[0047] prescription composition
[0048]
[0049] Preparation:
[0050] Prepare the prescribed amount of 0.1M citrate buffer solution with pH 6.2, accurately weigh the excipients in the prescribed amount, add an appropriate amount of phosphate buffer solution to dissolve, adjust the pH value to 5.5-6.5, and dilute to the volume for the recipe. The difference between the total volume of the solution and the stock solution of DSMb. Add the prescribed amount of DSMb stock solution into it, shake gently to mix, sterilize and filter with a microporous filter membrane with a pore size of 0.22 μm, subpackage, freeze-dry, stopper, and press the cap to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com