Nano metal catalyst and preparation method and application thereof
A nano-metal and catalyst technology, which is applied in the field of nano-metal catalysts and their preparation, can solve problems such as difficult to control hydrogenation, and achieve the effects of stable metal catalysts, high reactivity, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
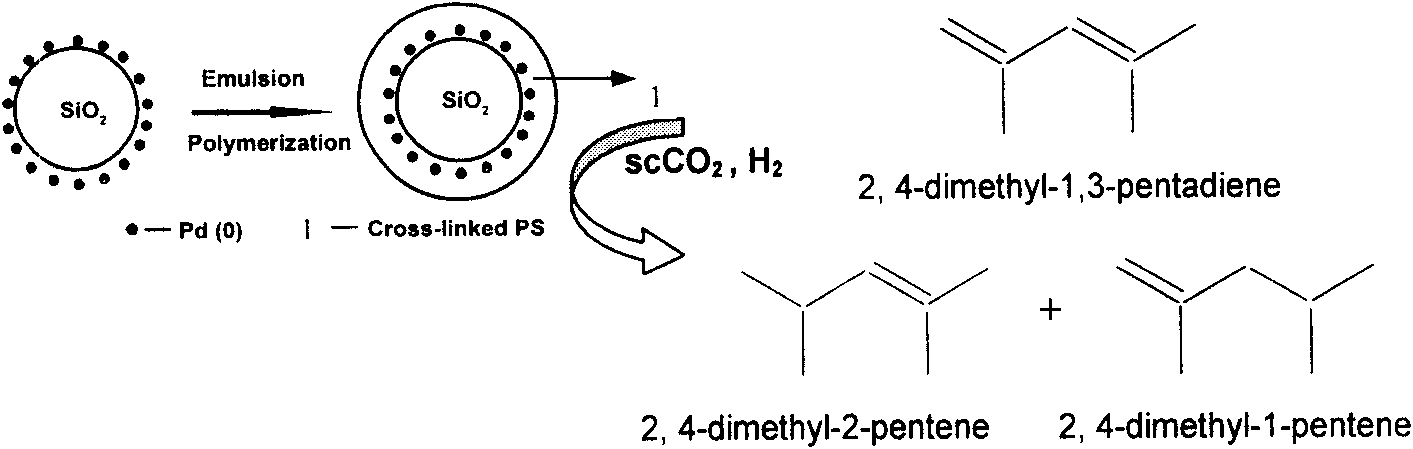
Image
Examples
Embodiment 1
[0016] Embodiment 1, preparation nano metal catalyst
[0017] 1) Preparation of monodisperse submicron SiO 2 particles
[0018] The ethanol solution of ethyl orthosilicate was quickly added to the ethanol solution of ammonia water, and in the final mixed solution, the molar ratio of ammonia:water:ethyl orthosilicate was 1:11:0.22. The resulting mixed solution was reacted under magnetic stirring for 4 hours at a reaction temperature of 30°C. The reacted suspension was centrifuged, washed three times with ethanol and water respectively, and dried at 100°C for 12 hours to obtain 230nm monodisperse submicron SiO 2 particles.
[0019] 2) SiO2 2 Amine functionalization of particles
[0020] 3 g SiO 2 The particles and 3.6 g of aminopropyltriethoxysilane were dispersed in 45 ml of toluene. After ultrasonic dispersion, the resulting suspension was refluxed at 110° C. for 24 hours with magnetic stirring. The refluxed suspension was centrifuged, washed three times with toluene an...
Embodiment 2
[0025] Embodiment 2, preparation nano metal catalyst
[0026] 1) Preparation of monodisperse submicron SiO 2 particles
[0027]The ethanol solution of ethyl orthosilicate was quickly added to the ethanol solution of ammonia water, and in the final mixed solution, the molar ratio of ammonia:water:ethyl orthosilicate was 1:11:0.22. The resulting mixed solution was reacted under magnetic stirring for 4 hours at a reaction temperature of 30°C. The reacted suspension was centrifuged, washed three times with ethanol and water respectively, and dried at 100°C for 12 hours to obtain 230nm monodisperse submicron SiO 2 particles.
[0028] 2) SiO2 2 Amine functionalization of particles
[0029] 3 g SiO 2 The particles and 3.6 g of aminopropyltriethoxysilane were dispersed in 45 ml of toluene. After ultrasonic dispersion, the resulting suspension was refluxed at 110° C. for 24 hours with magnetic stirring. The refluxed suspension was centrifuged, washed three times with toluene and...
Embodiment 3
[0034] Embodiment 3, preparation nano metal catalyst
[0035] 1) Preparation of monodisperse submicron SiO 2 particles
[0036] The ethanol solution of ethyl orthosilicate was quickly added to the ethanol solution of ammonia water, and in the final mixed solution, the molar ratio of ammonia:water:ethyl orthosilicate was 2:11:0.22. The resulting mixed solution was reacted under magnetic stirring for 4 hours at a reaction temperature of 30°C. The reacted suspension was centrifuged, washed three times with ethanol and water respectively, and dried at 100°C for 12 hours to obtain 500 nm monodisperse submicron SiO 2 particles.
[0037] 2) SiO2 2 Amine functionalization of particles
[0038] 3 g SiO 2 The particles and 3.6 g of aminopropyltriethoxysilane were dispersed in 45 ml of toluene. After ultrasonic dispersion, the resulting suspension was refluxed at 110° C. for 24 hours with magnetic stirring. The refluxed suspension was centrifuged, washed three times with toluene a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com