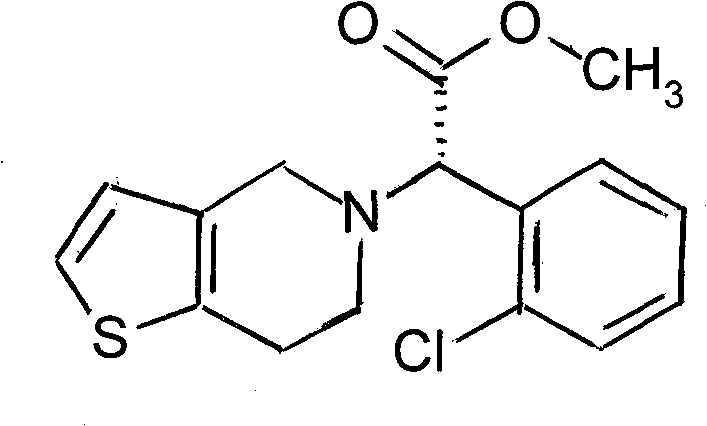
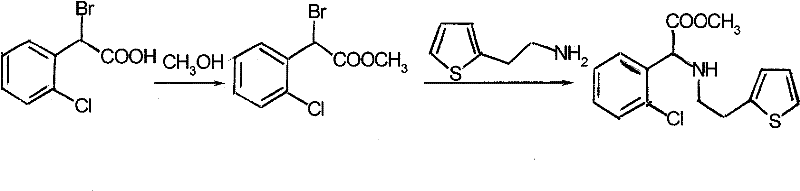
Method for synthesizing clopidogrel hydrogen sulfate intermediate by adopting solid acid catalytic esterification
A technology of clopidogrel hydrogen sulfate, acid catalyzed ester, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc. Difficulty in product separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Put 25g of α-bromo-o-chlorophenylacetic acid (0.1mol), 150ml of methanol (6 times), 12.5g of solid acid (0.5 times) into the reaction bottle, reflux for 8 hours, and add K after cooling down to room temperature 2 CO 3 Adjust pH=8, add 13.9ml (0.11mol) thienylethylamine, reflux reaction for 5 hours, add 100ml water and 125ml ethyl acetate for extraction, add hydrochloric acid to adjust pH=3, crystallize at 0°C for 10 hours. 29.5 g of α-(2-thienylethylamine)-2-chlorophenylacetic acid methyl ester hydrochloride was obtained, with a melting point of 174-175° C., an HPLC of 99.5%, and a yield of 85.01%.
Embodiment 2
[0022] Put 25g of α-bromo-o-chlorophenylacetic acid (0.1mol), 50ml of methanol (2 times), 2.5g of solid acid (0.1 times) into the reaction bottle, reflux for 16 hours, and add NaHCO after cooling down to room temperature 3 Adjust pH=4, add 10.1ml (0.08mol) thienylethylamine, reflux reaction for 10 hours, add 50ml water and 75ml ethyl acetate for extraction, add hydrochloric acid to adjust pH=5, crystallize at -20°C for 1 hour. 27.2 g of α-(2-thiopheneethylamine)-2-chlorophenylacetic acid methyl ester hydrochloride was obtained, with a melting point of 173-174° C., an HPLC of 98.3%, and a yield of 78.39%.
Embodiment 3
[0024] Put 25g of α-bromo-o-chlorophenylacetic acid (0.1mol), 250ml of methanol (10 times), 20g of solid acid (0.8 times) into the reaction bottle, reflux for 2 hours, and add Na 2 CO 3 Adjust pH=10, add 18.9ml (0.15mol) thienylethylamine, reflux reaction for 1 hour, add 200ml water and 250ml ethyl acetate for extraction, add hydrochloric acid to adjust pH=1, crystallize at 10°C for 20 hours. 29.1 g of α-(2-thiopheneethylamine)-2-chlorophenylacetic acid methyl ester hydrochloride was obtained, with a melting point of 174-175° C., an HPLC of 99.4%, and a yield of 83.86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com