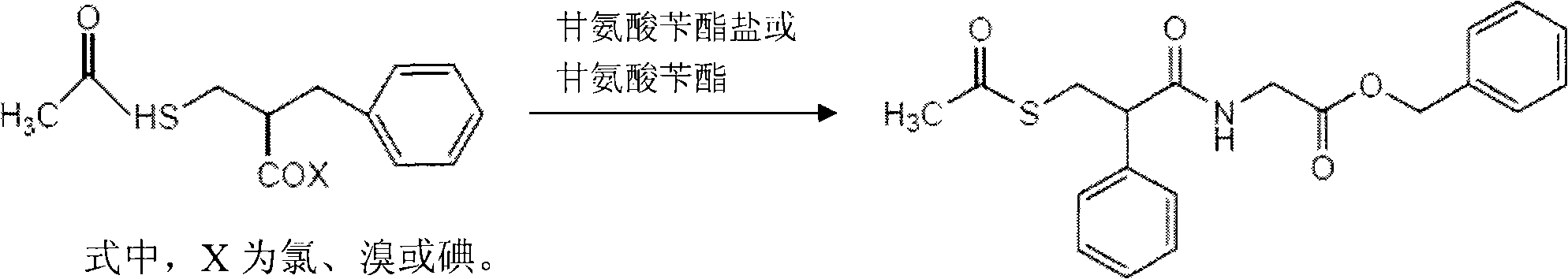
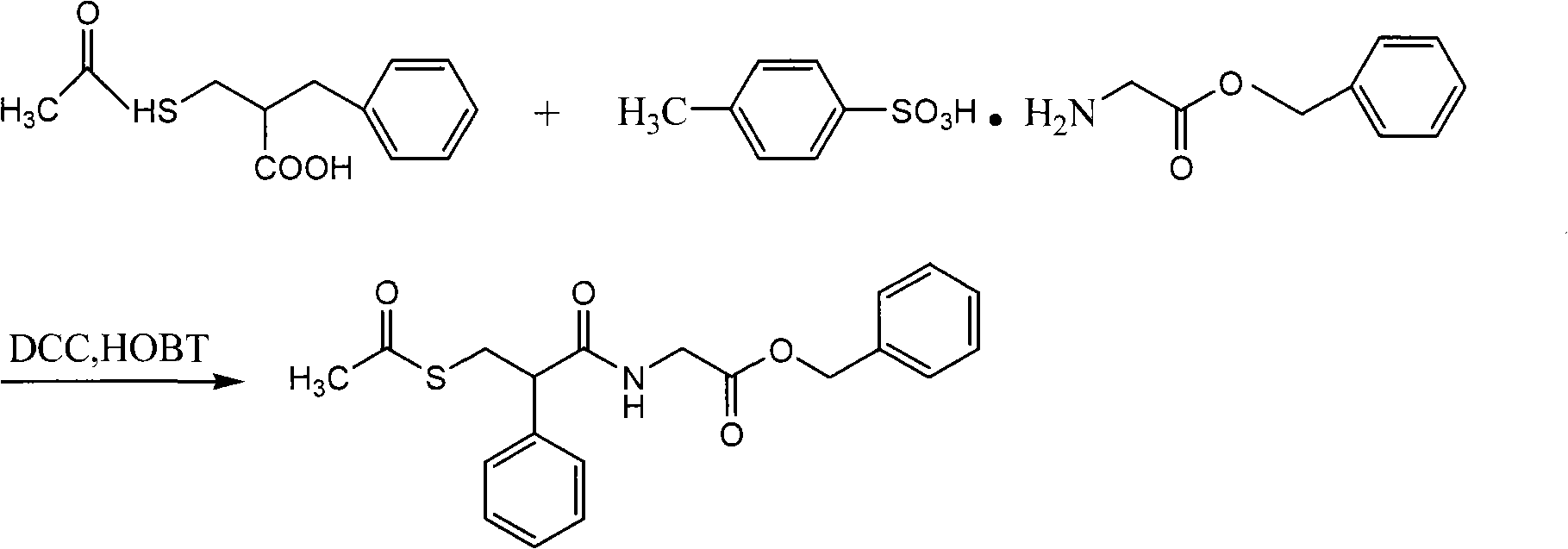
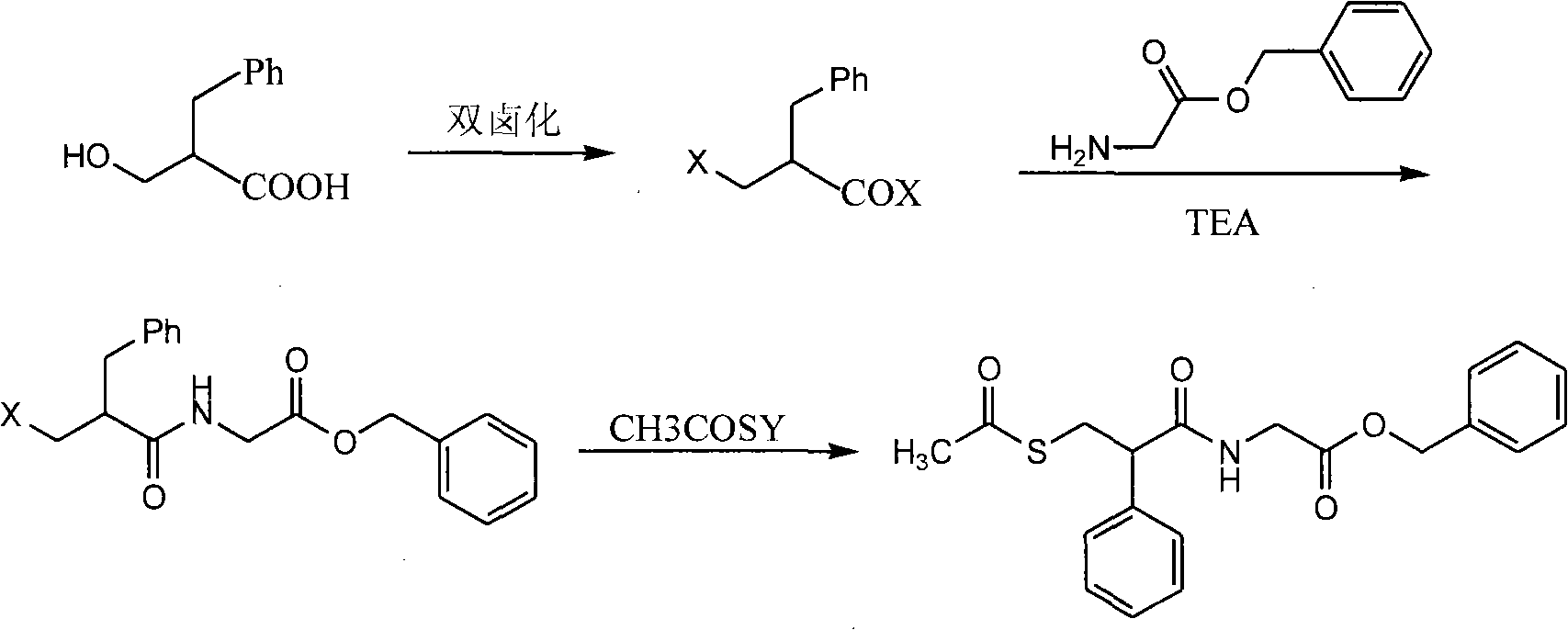
Preparation method of racecadotril
A technology for racecadotril and benzyl glycine, which is applied in the field of monothiocarboxylic acid, can solve the problems of high product impurity content, high price and high production cost of racecadotril, and achieves high yield and reaction process. short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] 1) Preparation of 2-benzyl-3-thioacetylpropionyl chloride
[0029] A 500ml three-necked reaction flask with a condensing tube is equipped with a tail gas absorption device, and 72g (0.3mol) of 2-benzyl-3-thioacetylpropionic acid, 57.1g (0.48mol) of thionyl chloride, and 300ml of toluene are added, slowly The temperature was raised to 35°C for 1 hour, and then the temperature was slowly raised to 70°C to continue the reaction for 3 hours. After the reaction was complete, 76.5 g of a light yellow oily substance was evaporated to dryness under reduced pressure.
[0030]
[0031] 2) Preparation of racecadotril
[0032] Add 860ml of dichloromethane and 101.1g (0.3mol) of benzyl glycine ester p-toluenesulfonate to a 2000ml three-necked flask, cool down in an ice bath to 0-5°C, add 125ml of triethylamine under stirring, after the addition is complete, add dropwise 76.5 g of 2-benzyl-3-thioacetylpropionyl chloride was prepared. After the dropwise reaction was c...
Embodiment 2
[0034]
[0035] 1) Preparation of 2-benzyl-3-thioacetylpropionyl chloride
[0036] A 500ml three-necked reaction flask with a condensing tube was installed with a tail gas absorption device, and 72g (0.3mol) of 2-benzyl-3-thioacetylpropionic acid, 38.1g (0.3mol) of oxalyl chloride, and 300ml of toluene were added, and the temperature was slowly raised to React at 35°C for 1 hour, then slowly raise the temperature to 60°C and continue to react for 3 hours. After the reaction is complete, the solvent is evaporated to dryness under reduced pressure to obtain 75.9 g of a light yellow oil.
[0037]
[0038] 2) Preparation of racecadotril
[0039] Add 860ml of dichloromethane and 101.1g (0.3mol) of benzyl glycine ester p-toluenesulfonate to a 2000ml three-necked flask, cool down in an ice bath to 0-5°C, add 124.2g of potassium carbonate while stirring, after the addition is complete, add step 1 dropwise ) 75.9 g of 2-benzyl-3-thioacetylpropionyl chloride prepared. After the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com