Suppository for treating mammalian endometritis and preparation method thereof
A technology for endometritis and mammals, applied in the direction of suppository delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of weak interaction of mucous membrane synovial fluid, unfavorable penetration into vaginal mucosa, Unsatisfactory bactericidal effect and other problems, to overcome the effect of irritation, strong bactericidal effect and long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
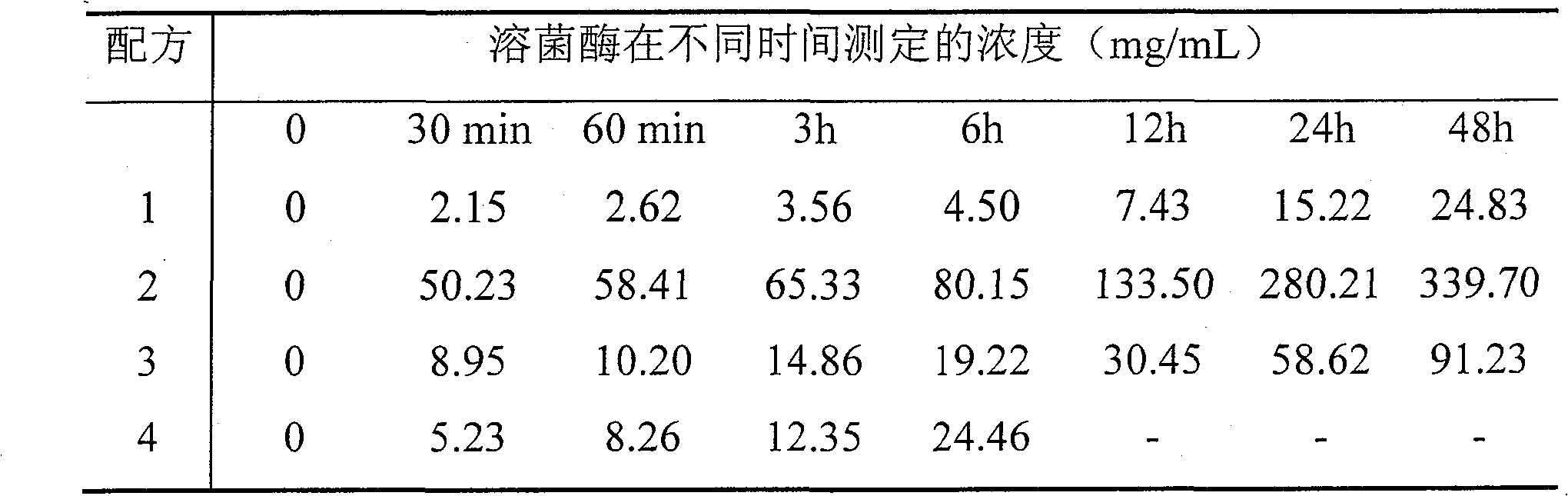
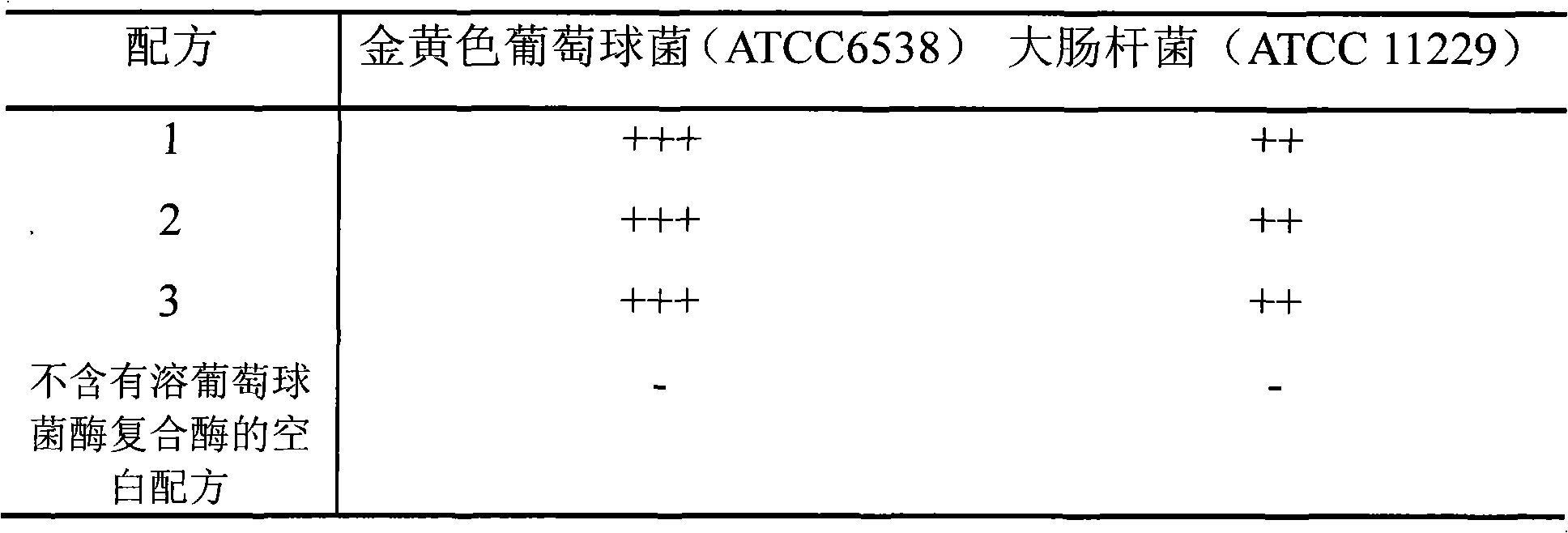
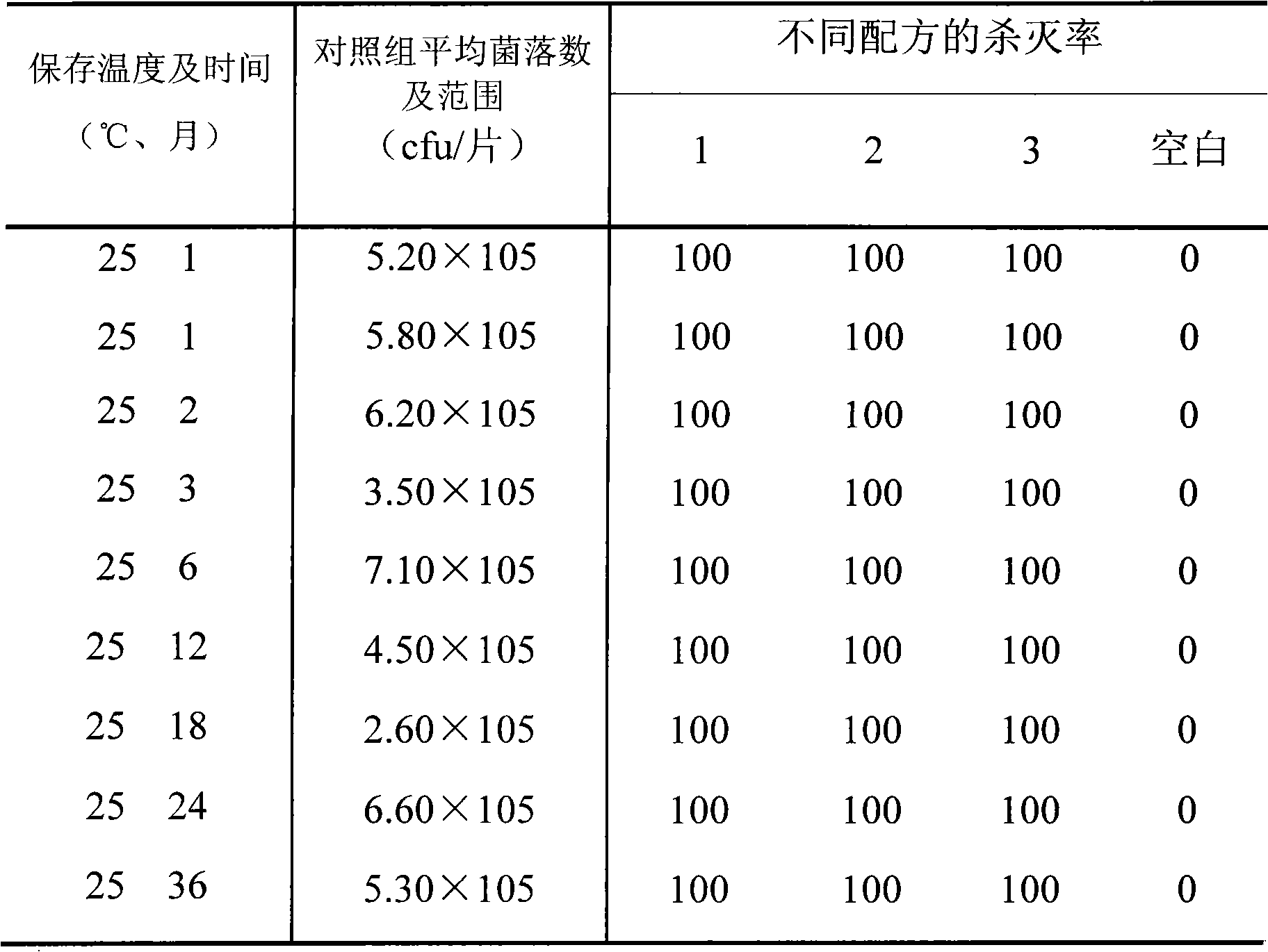
[0028] Embodiment 1 Lysostaphin suppository preparation method 1 (prescription 1)
[0029] 1. Composition and ratio
[0030] Lysostaphin 0.001%, lysozyme 0.1%, sodium alginate 0.1%, chlorhexidine 0.5%, polyoxyethylene stearate 99.299%.
[0031] 2. Preparation
[0032] (1) First, add 2 grams of lysozyme and 20 mg of lysostaphin, 2 grams of sodium alginate and 1 gram of chlorhexidine into 100 mL of aqueous solution and mix evenly, then add dropwise to 5% calcium chloride solution to form particles, and filter Microparticles were obtained by freeze-drying.
[0033](2) The microspheres are placed in normal saline, and placed at 37° C. for 6-8 hours to detect the amount of lysozyme in the solution (the detection method refers to literature: Liu Yuangang et al., Determination of the activity of lysozyme, Food Science and Technology, 2005, 10), The encapsulation efficiency of lysostaphin and lysozyme contained in the microspheres was calculated. Calculate the amount of microparti...
Embodiment 2
[0035] Embodiment 2 Lysostaphin suppository preparation method 2 (prescription 2)
[0036] 1. Composition and ratio
[0037] Lysostaphin 0.05%, lysozyme 2%, sodium alginate 1%, chlorhexidine 0.3%, polyoxyethylene stearate 96.65%.
[0038] 2. Preparation
[0039] (1) First, add 2 grams of lysozyme and 50 mg of lysostaphin, 1 gram of sodium alginate and 0.6 gram of chlorhexidine into 100 mL of aqueous solution and mix evenly, then add dropwise to 5% calcium chloride solution to form particles, and filter Microparticles were obtained by freeze-drying.
[0040] (2) Measure the encapsulation efficiency of the prepared microparticles as in Example 1, calculate the amount of microparticles to be added according to the encapsulation efficiency, and then take an appropriate amount of microparticles and add them to the dissolved polyoxyethylene stearate.
[0041] (3) Weigh 96.65g of polyoxyethylene stearate and heat it to 60°C to dissolve, then add 3.35g of the prepared particles int...
Embodiment 3
[0042] Embodiment 3 Lysostaphin suppository preparation method 3 (prescription 3)
[0043] 1. Composition and ratio
[0044] Lysostaphin 0.1%; Lysozyme 10%; Sodium alginate 1%, Polyoxyethylene stearate 88.9%.
[0045] 2. Preparation
[0046] (1) First, add 20 grams of lysozyme, 200 mg of lysostaphin, and 10 grams of sodium alginate into 200 mL of aqueous solution and mix evenly, then add dropwise to 5% calcium chloride solution to form particles, which are obtained by freeze-drying after filtration particle.
[0047] (2) Measure the encapsulation efficiency of the prepared microparticles as in Example 1, calculate the amount of microparticles to be added according to the encapsulation efficiency, and then take an appropriate amount of microparticles and add them to the dissolved polyoxyethylene stearate.
[0048] (3) Weigh 88.9 grams of polyoxyethylene stearate and heat it to 60°C to dissolve, then add 11.1 grams of the prepared microparticles to the dissolved polyoxyethyle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com