Process for production of EPA-enriched oil and DHA-enriched oil
一种油脂、工序的技术,应用在制造分别含有高浓度的二十碳五烯酸和二十二碳六烯酸浓缩油领域,能够解决双键数目多、氧化不稳定等问题,达到提高反应性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Lipase-catalyzed reaction of step a)
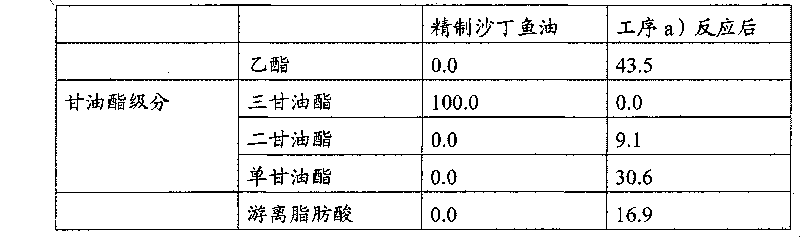
[0040] Add Lipaz QLM 1.49g (100unit / g), water 20.7g, magnesium oxide (Junzheng Chemical Co., Ltd. special grade Reagent 99% or more, powder) 30g (equivalent to 2.5% of oil), ethanol 207ml, stirred at 40°C for 16 hours. After the reaction, the solid was separated by filtration, and washed with 560 ml of 20% sulfuric acid and 200 ml of saline, respectively. Then, the water and ethanol remaining in the oil layer were distilled off to obtain 1.21 kg of oil. A small amount of the obtained oil was taken and the glyceride fraction was separated by preparative TLC, and after methylation, the fatty acid component was analyzed by gas chromatography. The analysis conditions are as follows (unless otherwise specified, subsequent analyzes by preparative TLC and gas chromatography were performed in the same manner).
[0041] TLC separation and preparation conditions, methyl esterification conditions;
[0042] 1 ml of hexane and 10 ml of s...
Embodiment 2
[0072] The amount of Lipozyme TLIM (Lipozyme TLIM) used in step 3) of Example 1 was changed to 5 mg (equivalent to 0.5% of the oil), and the reaction was carried out under the same conditions as in step (3) of Example 1. The results are shown in Table 7 and Table 8. Even when the amount of lipase used is reduced, DHA concentrated oil and EPA concentrated oil can be simultaneously obtained. In this reaction, the recovery rate of DHA in the glyceride fraction was 114.7%, and the recovery rate of EPA in the ethyl ester fraction was 58.9%. (The numerical value exceeding 100% in the recovery rate is due to the simple calculation method of the recovery rate calculation method this time. That is, the measured value of the FID used to calculate the recovery rate, because the greater the relative sensitivity of the molecular weight, the greater the relative sensitivity, so for DHA Fatty acid with such a high molecular weight can obtain a higher value. It is the same in the following e...
Embodiment 3
[0079] DHA concentrated oil and EPA concentrated oil were prepared using low-temperature isolated and purified sardine oil with high EPA concentration (EPA 29.0%, DHA 12.5%, manufactured by Nippon Suisan Co., Ltd.) as a raw material lipid. The reaction of step a) is to use 1.2Kg of refined sardine oil, 1.49g of Lipaz QLM, 20.7ml of water, 60g of magnesium oxide, and 207ml of ethanol, and react at 40°C for 16 hours. The treatment operation is carried out in the same manner as in Example 1. Then, under the same conditions as in Example 1, the thin-film distillation process was performed twice to obtain a glyceride fraction. Using 1 g of the oil obtained in step a), 10 μL of water, 20 mg of Lipsem TLIM, 25 mg of magnesium oxide, and 173 μL of ethanol, the reaction of step b) was carried out, and the reaction was carried out at 40° C. for 16 hours. The ethyl ester and glyceride fractions were separated and prepared by preparative TLC (the same conditions as in Example 1). Raw mat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com