Anti-human thyroglobulin monoclonal antibody and application thereof
A monoclonal antibody and immunoglobulin technology, applied in anti-animal/human immunoglobulin, applications, antibodies, etc., can solve the problem of ineffective activation of effector systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Preparation and Purification of Murine Monoclonal Antibody mTg-60
[0069] mouse immunization
[0070] Take Tg protein (1mg / mL) and fully emulsify with an equal volume of Freund's complete adjuvant, immunize 8-week-old female BALB / c mice by subcutaneous multi-point injection, and boost immunization with emulsified antigen in Freund's incomplete adjuvant 2 weeks apart The second time, 200 μg of purified protein antigen was injected intraperitoneally for booster immunization 3 days before cell fusion.
[0071] Cell fusion and screening of hybridoma cells
[0072] Splenocytes from immunized mice were fused with SP2 / O cells. After fusion, use HAT selection culture to culture in a 37°C, 50mL / L CO2 incubator. After fusion, half of the HAT selection medium was replaced on the 3rd and 6th day, and the HT selection medium was replaced on the 9th day. When the fused cells cover 1 / 4 to 1 / 2 of the wells, use Tg protein as the detection antigen, coat the 96-well plate with 10ug / m...
Embodiment 2
[0078] Functional identification of murine monoclonal antibody mTg-60
[0079] Functional detection was performed by solid-phase radioimmunoassay. The detection method has obtained the Shanghai Science and Technology Achievement Certificate (registration number: 931000948).
Embodiment 3
[0081] Preparation and purification of humanized monoclonal antibody hTg-37
[0082] (1) Preparation of humanized monoclonal antibody hTg-37
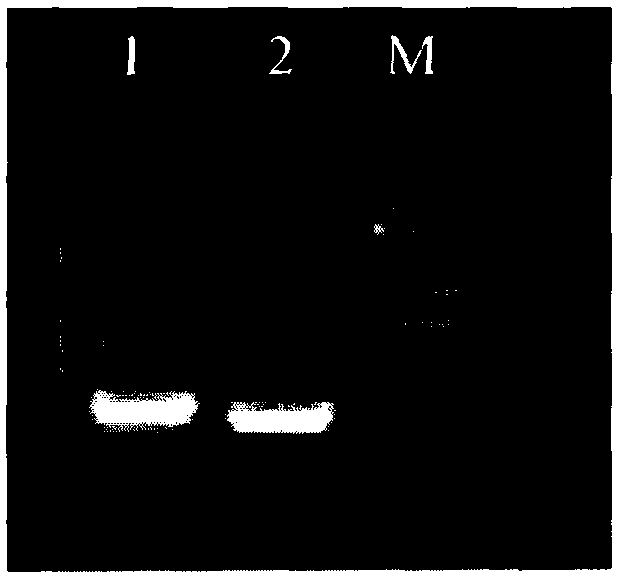
[0083] 1. Use PCR technology to amplify the heavy chain and light chain genes of the mouse monoclonal antibody MTg-60 hybridoma cell line, see figure 1 ;
[0084] 2. After gene sequencing, determine the sequences of heavy chain and light chain genes (SEQ ID NO: 13 and 14), design humanized primers (heavy chain SEQ ID NO: 9 and 10) and (light chain SEQ ID NO: 11 and 12);
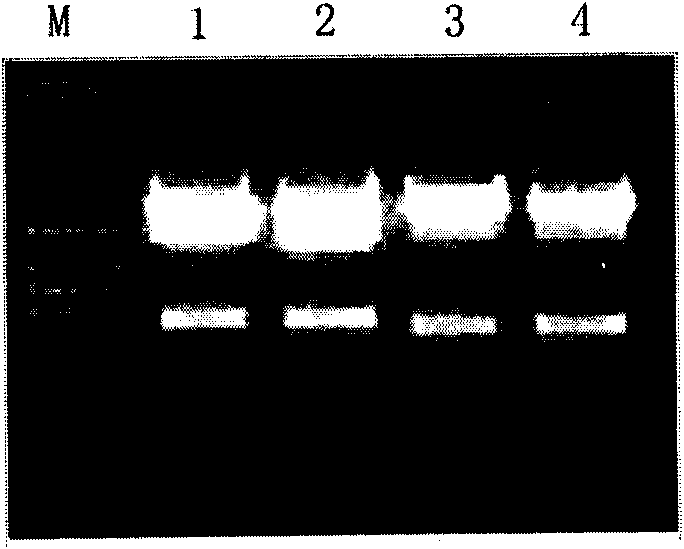
[0085] 3. Amplify the humanized monoclonal antibody hTg-37 heavy chain and light chain genes, see figure 2 ;
[0086] 4. Double digest the recovered humanized VH and VL gene PCR products BamH I and HindIII and clone them into the pCMV_CH and pVL_CL vectors that have been digested with the same enzymes, and transform Escherichia coli DH5α with the ligated products, and culture them upside down at 37°C overnight. Pick the clone, extract the plasmid, and identify i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com