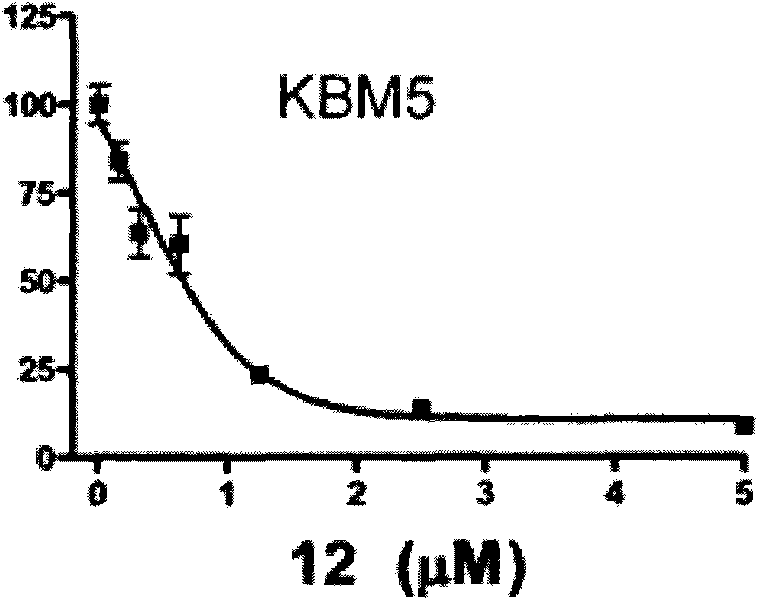
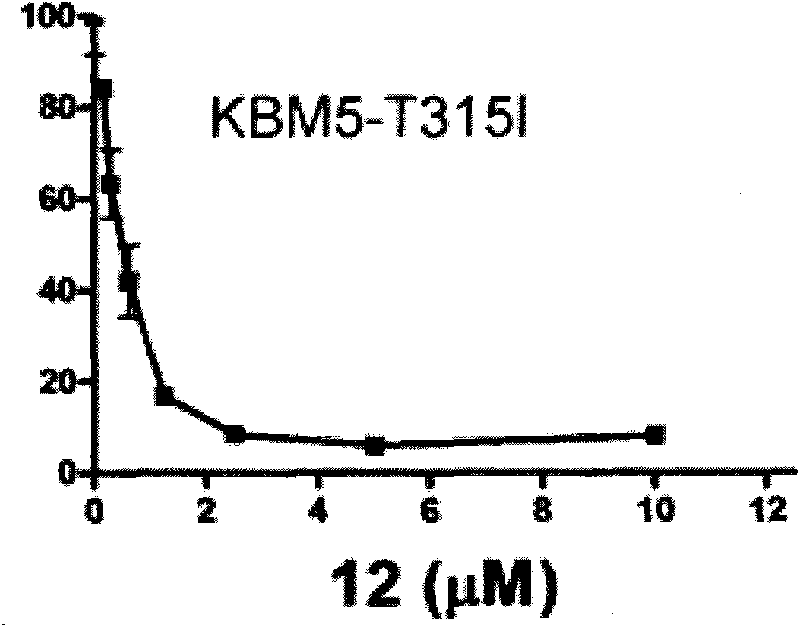
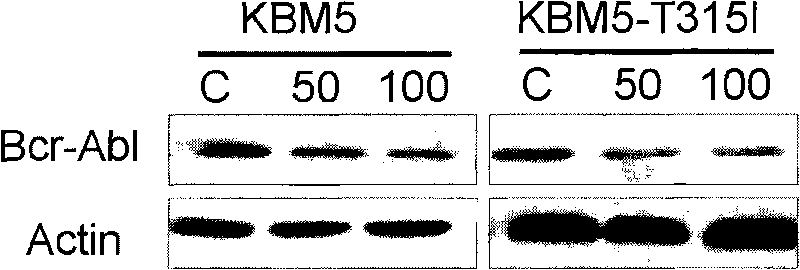
Triptolide derivative, and preparation method and application thereof
A technology of triptolide and derivatives, which can be used in drug combinations, pharmaceutical formulations, steroids, etc., can solve problems such as poor water solubility and toxicity, and achieve strong inhibitory activity, good application prospects, strong inhibition of leukemia cells and resistance to leukemia cells. Influenced by leukemia cell activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of triptolide methyl chloroacetate
[0037] Weigh triptolide (50mg, 0.139mmol) and dissolve it in 5ml chloroform, add 4-dimethylaminopyridine (85mg, 0.696mol) at -5°C and shake it for 0.5 hours, then slowly add the chlorine dissolved in chloroform dropwise. Acetyl chloride (0.039ml, 0.490mmol), slowly return to room temperature and stir for 24 hours, wash the reaction product with 5% dilute hydrochloric acid (volume percentage) and water for 2 to 3 times, dry over anhydrous magnesium sulfate, and distill under reduced pressure , silica gel column chromatography, collecting the eluent as V 乙酸乙酯 :V 石油醚 = 10:90 of the eluted product obtained, concentrated under reduced pressure to obtain 45 mg of a colorless transparent solid, which is triptolide chloroacetate, with a yield of 74.2% and a melting point of 122-124°C.
[0038] 1 H NMR (CDCl 3 , 300M Hz) δ5.30(s, 1H), 5.10(s, 1H), 4.67(s, 2H), 4.19(d, J=6.6Hz, 2H), 3.84(d, J=3Hz, 1H), 3.56(d...
Embodiment 2 4
[0039] The synthesis of embodiment 2 triptolide tetrahydropyrrolidone acetate
[0040] Weigh 10mg (0.029mmol) triptolide chloroacetate prepared in Example 1 and dissolve it in 1ml N,N-dimethylformamide, add 3.0mg (0.0348mmol) tetrahydropyrrole at 0°C, and weigh 4.8mg (0.0348mmol) Anhydrous K 2 CO 3 Add it to the above solution, react at room temperature 25°C for 24h, concentrate under reduced pressure, perform silica gel column chromatography, and collect the eluent as V 甲醇 :V 氯仿 =100:1 The obtained eluted product, the eluted product was concentrated under reduced pressure to obtain 7.4 mg of a colorless solid, which is triptolide of tetrahydropyrrolidone acetate in this example, and the yield was 74%. mp 152-154°C.
[0041] 1 H NMR (CDCl 3 , 300M Hz) δ5.13(s, 1H), 4.68(s, 2H), 4.19(d, J=7.2Hz, 2H), 3.89(d, J=3.3Hz, 1H), 3.55(d, J= 3.3Hz, 1H), 3.48(d, J=5.4Hz, 1H), 2.75(m, 1H), 2.35(m, 2H), 2.35(m, 2H), 2,20(m, 2H), 1.93( m, 2H), 1.90(m, 2H), 1.90(m, 2H), 1.85(m, 1H), ...
Embodiment 4
[0045] The synthesis of embodiment 4 morphine morpholino acetate triptolide
[0046] The specific process is the same as in Example 2, and the molar ratio of each reaction substance is also the same as in Example 2, with a yield of 75%, a colorless solid. mp 98-99°C.
[0047] 1 H NMR (CDCl 3 , 300M Hz) δ5.13(s, 1H), 4.67(s, 2H), 3.83(d, J=3.0Hz, 1H), 3.76(t, J=4.5Hz, 2H), 3.76(t, J= 4.5Hz, 2H), 3.54(d, J=3.0Hz, 1H), 3.48(dd, J=5.7Hz, 1H), 3.35(q, J=16.5Hz, 28.2Hz, 2H), 2.68(m, 1H ), 2.64(m, 2H), 2.64(m, 2H), 2.17(m, 2H), 1.90(m, 2H), 1.56(m, 1H), 1.45(m, 2H), 1.26(s, 1H) , 1.21(m, 1H), 1.01(s, 3H), 0.98(d, J=6.9Hz, 3H), 0.86(d, J=6.9Hz, 3H). 13 C NMR (CDCl3, 75M Hz) δ173.0, 169.5, 159.7, 125.5, 71.2, 69.9, 66.9, 66.9, 63.6, 63.2, 61.3, 59.7, 59.6, 55.5, 55.1, 53.1, 53.1, 40.4, 35.8, 29.9, 28.3, 23.6, 17.6, 17.2, 16.8, 13.8. HREIMS m / z 487.2200 (calcd for C 26 h 33 o 8 N 1 , 487.2201).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com