Method for preparing p-diacetylbenzene by biomimetic catalytic oxidation of p-diethylbenzene with oxygen
A technology of diacetylbenzene and p-diethylbenzene, which is applied in the field of biomimetic catalytic oxygen oxidation of p-diethylbenzene to prepare p-diacetylbenzene, can solve the problems of wasting raw materials, high air flow rate, and increased operating costs, and speed up production Cycle, gas flow rate savings, and operating cost reduction effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] In a 100mL three-necked flask, add 13.474g p-diethylbenzene, 1ppm (0.07mg) tetraphenyliron porphyrin (i.e. R in formula (I) 11 for H, R 12 for H, R 13 for H, M 1 Fe), 10ppm (0.86mg) four-(p-chlorophenyl) cobalt porphyrin (that is, R in the formula (I) 11 for H, R 12 for H, R 13 for Cl, M 1 For Co), oxygen was introduced at a flow rate of 40 mL / min, the reaction was initiated at 150°C, and the reaction was carried out at 110°C for 18h. The reacted mixture was frozen, centrifugally filtered, and then recrystallized with ethanol to obtain p-diacetylbenzene. The conversion rate of p-diethylbenzene was 80.4%, the yield of p-diacetylbenzene was 59.8%, and the purity was 99.1%.
Embodiment 2
[0046] In a 100mL three-necked flask, add 13.432g p-diethylbenzene, 5ppm (0.38mg) tetrakis-phenylmanganese porphyrin chloride (i.e. R in formula (II) 21 for H, R 22 for H, R 23 for H, M 2 is Mn, X is Cl), 5ppm (0.44mg) tetrakis-(o-methoxyphenyl) cobalt porphyrin chloride (that is, R in the formula (II) 21 for OCH 3 , R 22 for H, R 23 for H, M 2 is Co, X is Cl), oxygen was introduced at a flow rate of 40 mL / min, the reaction was initiated at 150°C, and the reaction was carried out at 120°C for 16h. The reacted mixture was frozen, centrifugally filtered, and then recrystallized with ethanol to obtain p-diacetylbenzene. The conversion rate of p-diethylbenzene was 85.1%, the yield of p-diacetylbenzene was 62.2%, and the purity was 99.3%.
Embodiment 3
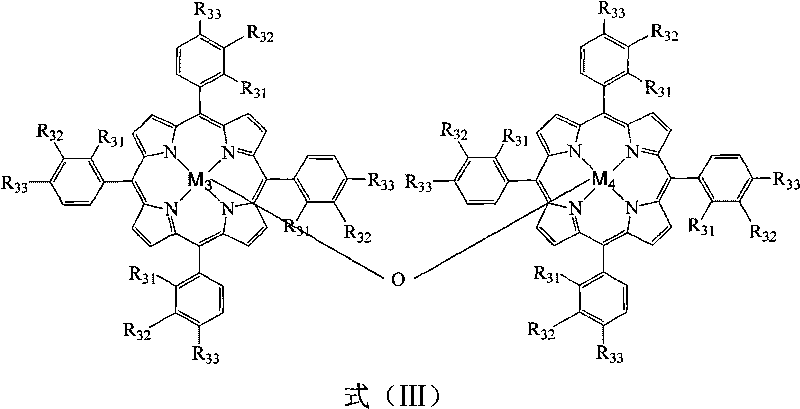
[0048] In a 100mL three-necked flask, add 13.487g p-diethylbenzene, 1ppm (0.15mg) μ-oxygen-binuclear tetra-phenyliron porphyrin (that is, R in formula (III) 31 for H, R 32 for H, R 33 for H, M 3 , M 4 Fe), 5ppm (0.88mg) μ-oxygen-binuclear four-(p-chlorophenyl) cobalt porphyrin (that is, R in formula (III) 31 for H, R 32 for H, R 33 for Cl, M 3 , M 4 For Co), oxygen was introduced at a flow rate of 50 mL / min, the reaction was initiated at 150 °C, and the reaction was carried out at 100 °C for 16 h. The reacted mixture was frozen, centrifugally filtered, and then recrystallized with ethanol to obtain p-diacetylbenzene. The conversion rate of p-diethylbenzene was 75.3%, the yield of p-diacetylbenzene was 50.6%, and the purity was 99.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com