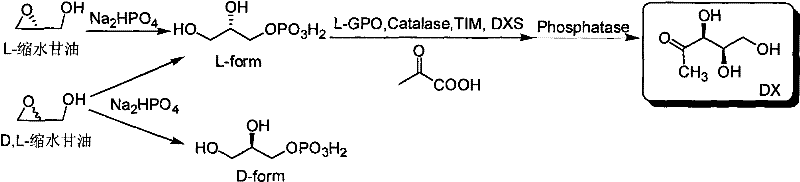
Method for preparing 1-deoxy-D-xylulose with chemical method-enzymatic method
A technology for enzymatic preparation of xylulose, applied in chemical instruments and methods, deoxygenated/unsaturated sugars, organic chemistry, etc., can solve the problems of expensive reaction raw materials, cumbersome reaction steps, and low total yield, and achieve purification steps Simple and easy to implement, low price, and the effect of increasing conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Take 148mg Na 2 HPO 4 In a 15mL centrifuge tube, add 800μl ultrapure water to dissolve it, then add 0.5mmol glycidol or 0.25mmol (R)-glycidol, and react in a water bath at 100°C for 3h; after the centrifuge tube is cooled to room temperature, adjust the pH to 7.5, Add catalase 180units, L-GPO 3units, sodium pyruvate 55mg, TIM 25units, ThPP 2mM, MgCl 2 10mM, β-mercaptoethanol 5mM, DXS 500μg, the total reaction volume is 1mL, and react at 37°C for 12 hours; during the reaction process, use paper chromatography to detect the degree of reaction, the medium is qualitative filter paper commonly used in laboratories, and the developer is 1M acetic acid Ammonium: 99% ethanol: EDTA-Na 2 =70:30:1 (v / v / v), the developer is 10% ethylenediamine sulfate aqueous solution. After the reaction is complete, add 20 units of alkaline phosphatase to the reaction mixture and incubate at 37°C for 4 hours; remove the moisture in the reaction mixture by vacuum rotary evaporation, add (500 μl×...
Embodiment 2
[0040] Take 100mg Na 2 Add 500μl ultrapure water to dissolve HPO4 in a 15mL centrifuge tube, then add 0.25mmol (rac)-glycidol or 0.125mmol (R)-glycidol, and react in a 50°C water bath for 2 hours; after the reaction, the centrifuge tube After cooling to room temperature, adjust the pH to 7.0, add Catalase 100units, L-GPO 1units, sodium pyruvate 30mg, TIM 10units, ThPP 1mM, MgCl 2 2mM, β-mercaptoethanol 1mM, DXS 200μg, total reaction volume 600μL, 30°C for 6 hours; during the reaction, use paper chromatography to detect the extent of the reaction, the medium is qualitative filter paper commonly used in laboratories, and the developer is 1M acetic acid Ammonium: 99% ethanol: EDTA-Na 2 =70:30:1 (v / v / v), the developer is 10% ethylenediamine sulfate aqueous solution. After the reaction is complete, add 5 units of alkaline phosphatase to the reaction mixture and incubate at 30°C for 2 hours; remove the moisture in the reaction mixture by vacuum rotary evaporation, add (500 μl×2) m...
Embodiment 3
[0042] Take 200mg Na 2 HPO 4 In a 15mL centrifuge tube, add 1000μl ultrapure water to dissolve it, then add 1mmol (rac)-glycidol or 0.5mmol (R)-glycidol, and react in a water bath at 100°C for 5h; after the reaction, cool the centrifuge tube to After room temperature, adjust the pH to 8.0, add Catalase 200units, L-GPO20units, sodium pyruvate 60mg, TIM 30units, ThPP 5mM, MgCl 2 10mM, β-mercaptoethanol 5mM, DXS 800μg, the total reaction volume is 1.5mL, and react at 40°C for 14 hours; during the reaction process, the degree of reaction is detected by paper chromatography, the medium is qualitative filter paper commonly used in the laboratory, and the developer is 1M Ammonium acetate: 99% ethanol: EDTA-Na 2=70:30:1 (v / v / v), the developer is 10% ethylenediamine sulfate aqueous solution. After the reaction is complete, add 30 units of alkaline phosphatase to the reaction mixture and incubate at 30°C for 4 hours; remove the moisture in the reaction mixture by vacuum rotary evapor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com