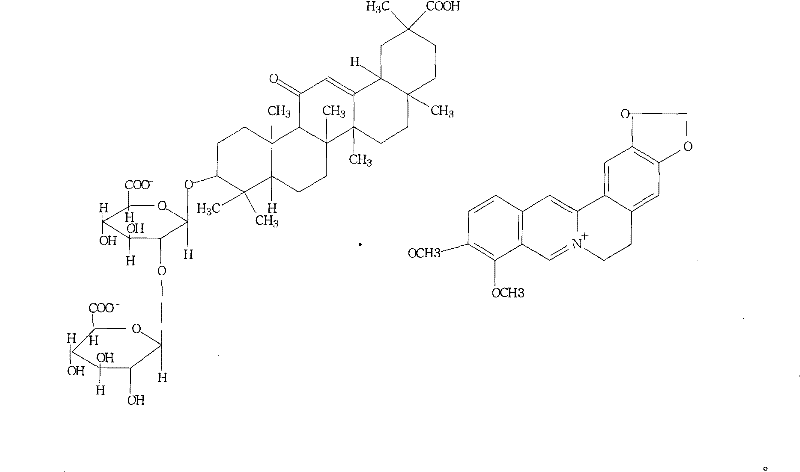
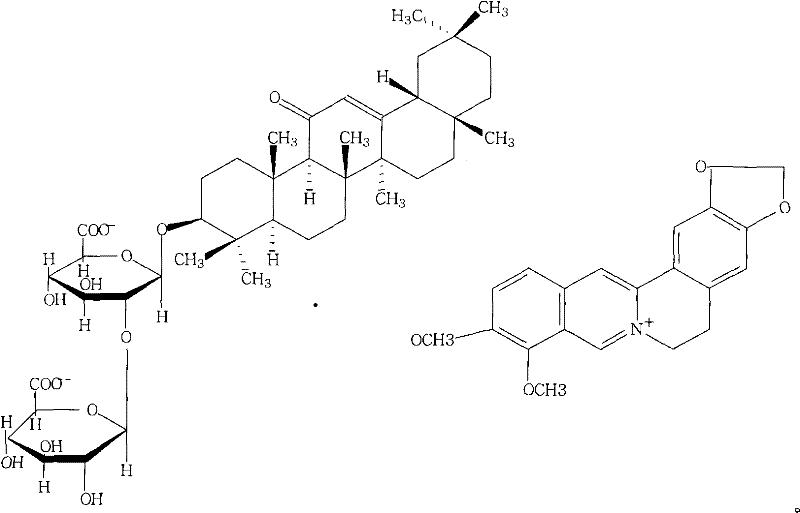
Berberine glycyrrhizic acid enantiomer salt and preparation method and usage thereof
A technology of glycyrrhizic acid and berberine, which is applied in the preparation of sugar derivatives, chemical instruments and methods, and pharmaceutical formulations, and can solve the problems of insufficient clinical efficacy and therapeutic range of berberine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] What this embodiment provides is the preparation method of berberine 18β-glycyrrhizinate:
[0026] Take about 60g of berberine and dissolve it in hot water; add 100g of anion exchange resin (Amberite IR-400) after cooling to room temperature, stir for 1 hour, filter, remove the resin, and put the filtrate into a solution containing 74g of 18β-monoammonium glycyrrhizinate , adjust the pH to about 4.0, stir for 30 minutes, let stand, refrigerate for 24 hours, filter, wash the precipitate twice with a small amount of ice water, drain, dry in vacuum at 60°C, add ethanol to dissolve, and filter. The alcohol solution was vacuum-dried below 60°C to obtain 87g of brown-yellow powder. The yield is 65%.
[0027] mp: 215-218°C
[0028] [C] D20+47.4° (c=0.707, methanol)
[0029]
Embodiment 2
[0031] What this embodiment provides is the preparation method of berberine 18a-glycyrrhizinate:
[0032] Take about 60g of berberine, dissolve it in hot water; take another 74g of 18a-monoammonium glycyrrhizinate, dissolve it in water, adjust the pH to about 4, add it into the above solution under stirring at room temperature, stir for 30min, let stand, and refrigerate for 24 hours, filtered, the precipitate was washed twice with a small amount of ice water, sucked dry, dried in vacuum at 60°C, dissolved in ethanol, and filtered. The alcohol solution was vacuum-dried below 60°C to obtain 94g of brown yellow powder. The yield is 70%.
[0033] mp: 248-254°C
[0034][C] D20+18.3° (c=0.707, methanol)
[0035]
Embodiment 3
[0037] This example describes the effect of compounds provided by the present invention on the blood sugar of alloxan diabetic mice:
[0038] Alloxan was used to create a hyperglycemia model in mice, and the mice with blood glucose > 11mmol / l were randomly divided into groups, model control group, 18β-glycyrrhizinate berberine salt, 18a-glycyrrhizinate berberine salt, and positive control group were given Normal saline, 18β-glycyrrhizinate berberine salt, 18a-glycyrrhizinate berberine salt, berberine, once a day, for 14 consecutive days, fasting for 12 hours after the last administration, without water, test fasting blood sugar, observe medication Changes in blood sugar before and after.
[0039] Table 1 Blood glucose before and after alloxan administration in mice
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com