Method for determining content of compound by utilizing relative correction factor
A technology of relative correction factors and compounds, applied in measurement devices, instruments, scientific instruments, etc., can solve problems such as time-consuming, and achieve accurate results and simple methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
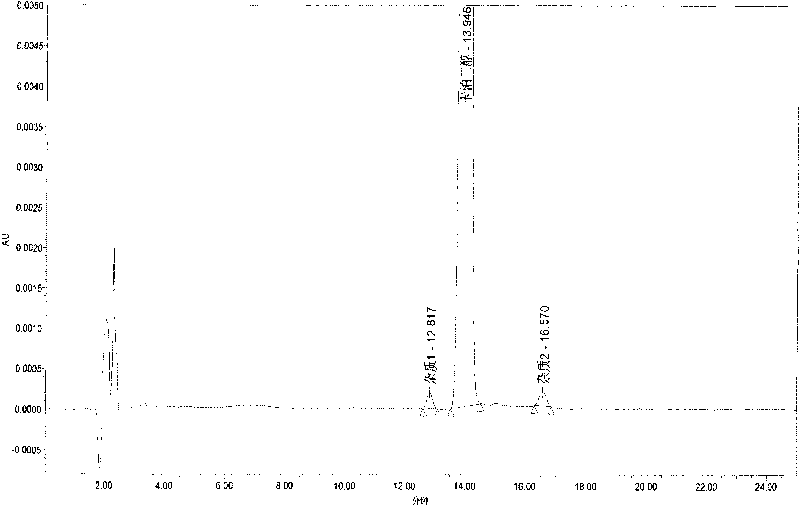
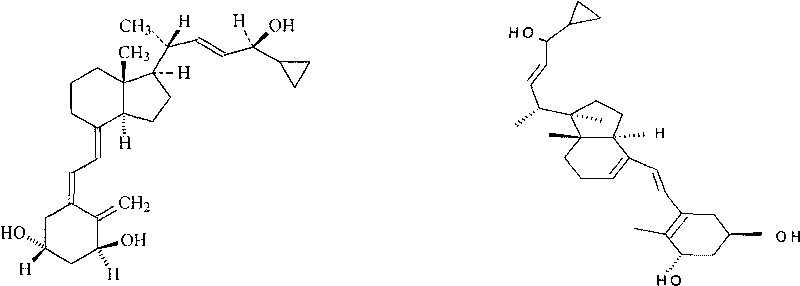
[0048] The mensuration (chromatographic condition 1) of calcipotriol relative correction factor before embodiment 1
[0049] 1) Preparation of standard solution
[0050] Take calcipotriol standard 10mg, put it in a 50ml measuring bottle, add 10ml of methanol to dissolve, add extraction solvent to dilute to the mark, shake well, take 10.0ml and put it in 100ml, add extraction solvent to dilute to the mark, shake well, take appropriate amount respectively Put it in a vial, seal it with a cap, put it in a water bath at 75°C for 30, 60, and 120 minutes, take it out, and cool it down quickly.
[0051] 2) Take the above-mentioned standard solution kept in the constant temperature water bath at 75°C at different time points, and inject 20 μl of it according to the chromatographic condition 1 respectively. Record the chromatogram, measure the pre-calcipotriol peak area and the calcipotriol peak area respectively, and calculate the relative correction factor. The measurement results a...
Embodiment 2
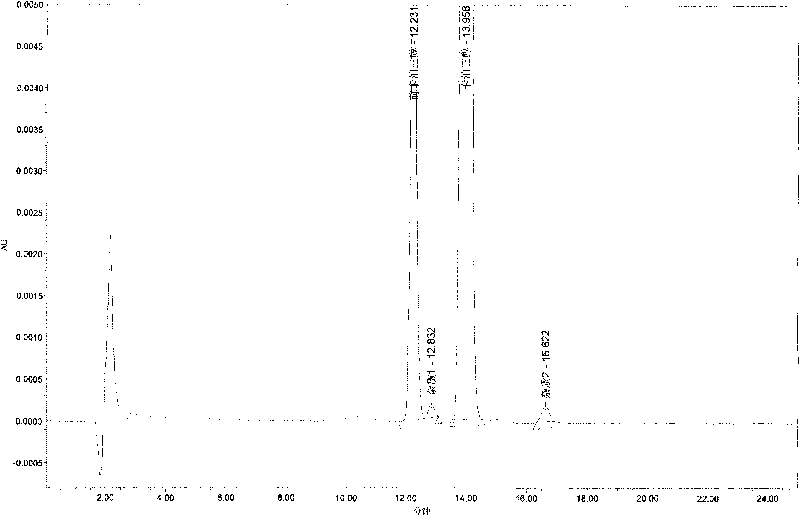
[0059] The mensuration of calcipotriol relative correction factor (chromatographic condition 2) before embodiment 2
[0060] 1) Preparation of standard solution
[0061] Take calcipotriol standard 10mg, put it in a 50ml measuring bottle, add 10ml of methanol to dissolve, add extraction solvent to dilute to the mark, shake well, take 10.0ml and put it in 100ml, add extraction solvent to dilute to the mark, shake well, take appropriate amount respectively Put it in a vial, seal it with a cap, put it in a water bath at 75°C for 30, 60, and 120 minutes, take it out, and cool it down quickly.
[0062] 2) Take the above-mentioned standard solution kept at different time points in the constant temperature water bath at 75°C, and inject 20 μl of it according to the chromatographic condition 2 respectively. Record the chromatogram, measure the calcipotriol peak area and the calcipotriol peak area respectively, and calculate the relative correction factor. The measurement results are s...
Embodiment 3
[0067] Example 3 Calcipotriol Ointment Marked Content Determination
[0068] 1. Test samples:
[0069] Samples 1 to 3: calcipotriol ointment (batch numbers: 20080301, 20080302, 20080303, manufacturer: Chongqing Huabang Pharmaceutical Co., Ltd.).
[0070] Sample 4: Calcipotriol ointment (Lot No.: EA8695, Leo Pharmaceuticals Ltd., Ireland).
[0071] Ointment blank base (batch number: 20080201, manufacturer: Chongqing Huabang Pharmaceutical Co., Ltd.).
[0072] Calcipotriol standard substance: Weigh two parts and measure twice for each part.
[0073] Samples: Weigh two parts of each sample, and measure each sample once.
[0074] 2. Determination of labeled content of calcipotriol ointment by relative correction factor method
[0075] (1) Chromatographic conditions: same as chromatographic conditions 1
[0076] (2) Preparation of internal standard stock solution Take 20 mg of methyltestosterone, put it in a 100ml measuring bottle, add extraction solvent to dissolve and dilute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com