A kind of method that hydrophobic rhenium ionic liquid catalyzes olefin epoxidation
An ionic liquid and hydrophobic technology, applied in the direction of chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem of high price, complicated process, non-recyclable reuse, etc. problems, to achieve the effect of protecting the environment and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: the method for cyclopentene epoxidation catalyzed by hydrophobic rhenium ionic liquid
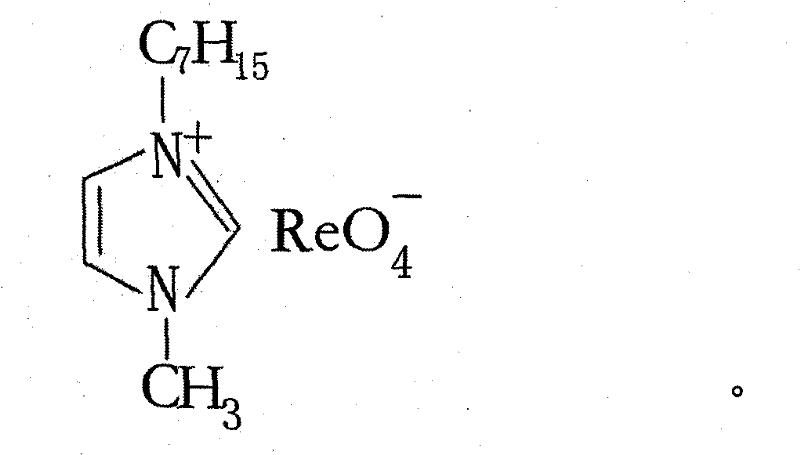
[0018] (1) Preparation of hydrophobic rhenium ionic liquid
[0019] Take 50g of heptylimidazole bromide, dissolve it in 300ml of deionized water, carry out ion exchange in hydroxyl resin, collect the effluent, add it to 1000ml of 10% (w / w) ammonium rhenate aqueous solution, and heat it at 70~90℃ Stir for 3 to 5 hours, remove water by rotary evaporation, add anhydrous methanol and acetonitrile mixed solution after cooling, seal and stir vigorously for 10 minutes, place at -30 to -40°C for 10 to 15 hours, distill under reduced pressure to remove methanol and acetonitrile , vacuum dried.
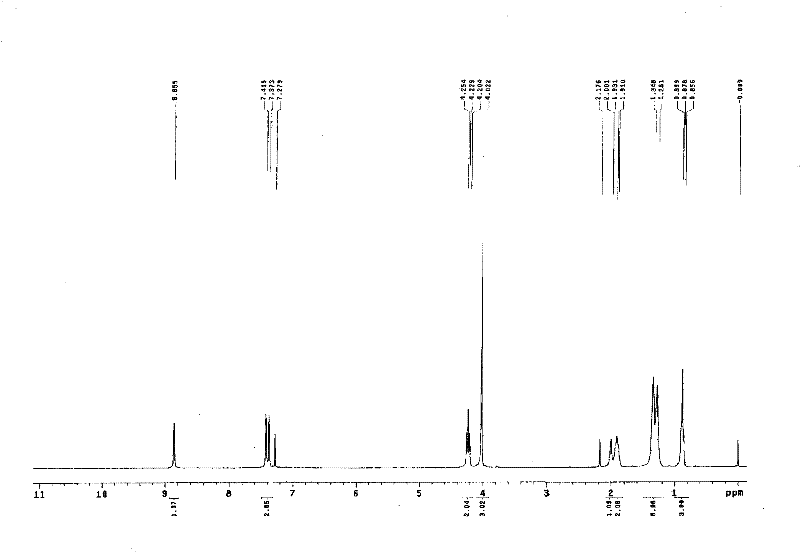
[0020] NMR characterization of hydrophobic rhenium ionic liquid figure 1 .
[0021] (2) Hydrophobic rhenium ionic liquid catalyzed cyclopentene epoxidation method
[0022] Preheat the hydrophobic rhenium ionic liquid with an oil bath to a reaction temperature of 50°C, add carbamide p...
Embodiment 2
[0023] Example 2 Cyclohexene epoxidation catalyzed by hydrophobic rhenium ionic liquid
[0024] (1) Preparation of hydrophobic rhenium ionic liquid
[0025] Same as Example 1
[0026] (2) Cyclohexene epoxidation catalyzed by hydrophobic rhenium ionic liquid
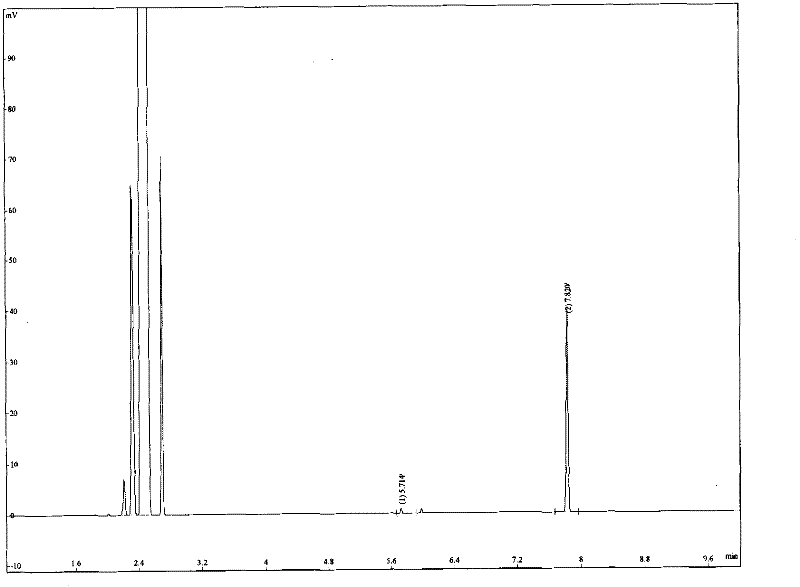
[0027] Preheat the hydrophobic rhenium ionic liquid with an oil bath to a reaction temperature of 60°C, add carbamide peroxide, the mole number of carbamide peroxide is 1.5 times the mole number of cyclohexene, and after mixing evenly, add cyclohexene, in which ions The mole number of the liquid is 80% of the mole number of cyclohexene, mechanically stirred under normal pressure, and the reaction time is controlled at 3 hours. After the reaction was finished, the product and substrate were extracted with n-hexane, the conversion rate of the upper layer was measured by gas chromatography, and its quantitative index was confirmed by the internal standard method to obtain a single product, which was the epoxide of the alke...
Embodiment 3
[0028] Example 3 Hydrophobic rhenium ionic liquid catalyzes the epoxidation of 1-hexene
[0029] (1) Preparation of hydrophobic rhenium ionic liquid
[0030] Same as Example 1
[0031] (2) Hydrophobic rhenium ionic liquid catalyzed the epoxidation of 1-hexene
[0032] Preheat the hydrophobic rhenium ionic liquid with an oil bath to a reaction temperature of 65°C, add carbamide peroxide, the molar number of carbamide peroxide is 2.5 times the molar number of 1-hexene, after mixing evenly, add 1-hexene, Wherein the mole number of the ionic liquid is 100% of the mole number of 1-hexene, mechanically stirred under normal pressure, and the reaction time is controlled at 3 hours. After the reaction was finished, the product and substrate were extracted with n-hexane, and the conversion rate of the upper layer was measured by gas chromatography, and its quantitative index was confirmed by the internal standard method to obtain a single product, which was the epoxide of the alkene, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com