Medicine composition of naloxone hydrochloride and polyethylene glycol and preparation method thereof
A technology of naloxone hydrochloride and polyethylene glycol, which is applied in the field of medicine, can solve problems such as unstable light exposure and difficulty in mastering clinical dosage, and achieves difficult degradation, safe and effective clinical medication, convenient dosage and effective dosage. The effect of dosing on mastery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0049] Hereinafter, the above-mentioned content of the present invention will be further described in detail through specific implementations in the form of examples. However, it should not be understood that the scope of the above-mentioned subject of the present invention is limited to the following embodiments. All technologies implemented based on the foregoing content of the present invention belong to the scope of the present invention.
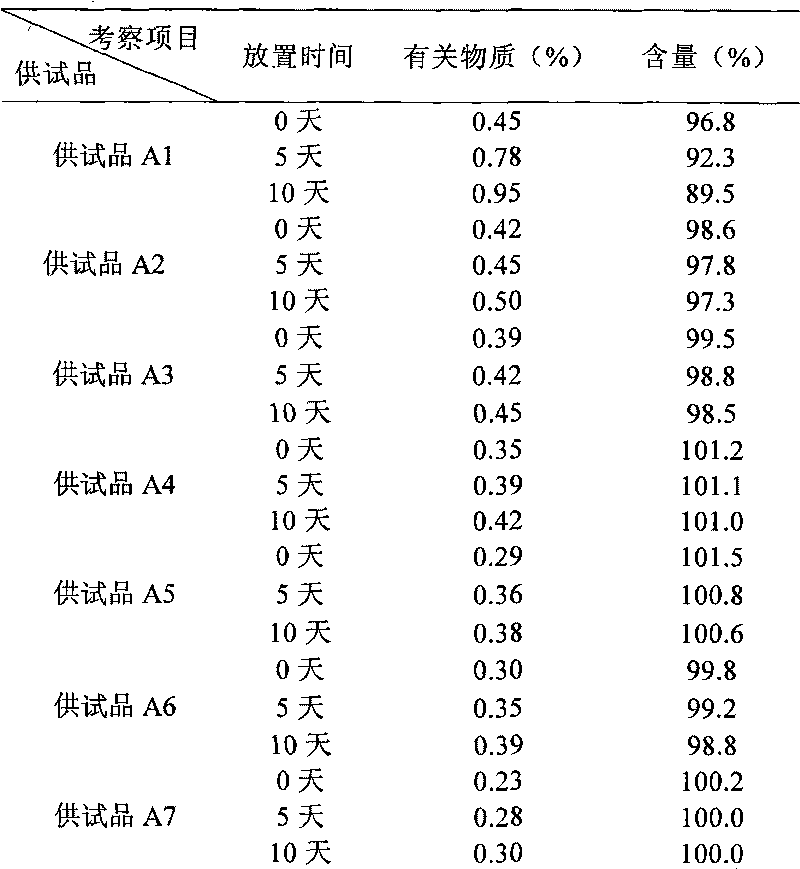
[0050] Example Preparation of the composition injection of the present invention
[0051] Prescription 1
[0052] Naloxone Hydrochloride 2g
[0053] Polyethylene glycol 2g
[0054] Add water for injection to 2000ml
[0055]
[0056] 1000 pieces in total
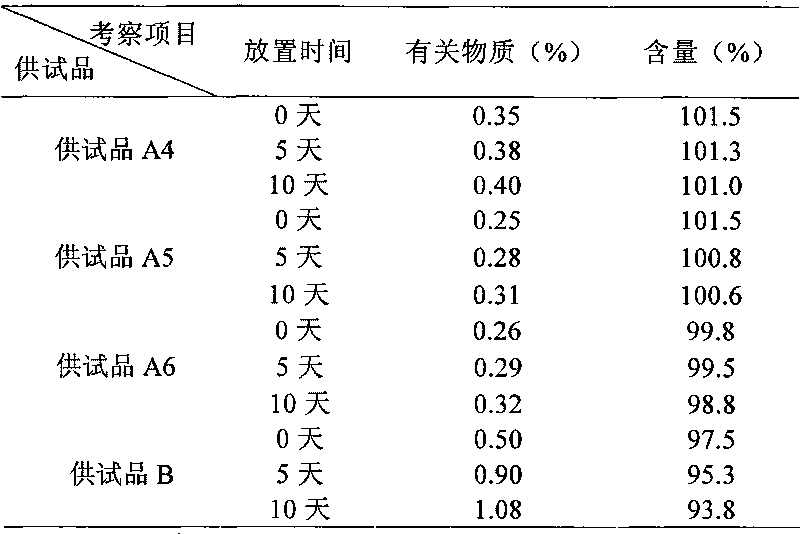
[0057] Prescription 2
[0058] Naloxone Hydrochloride 2g
[0059] Polyethylene glycol 1g
[0060] Add water for injection to 2000ml
[0061]
[0062] 1000 pieces in total
[0063] Preparation method of prescription 1, 2:
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com