Creatine phosphate sodium freeze-dried preparation and method for preparing same
A technology of creatine phosphate sodium and freeze-dried preparations, which is applied in the field of myocardium protection drug creatine phosphate sodium freeze-dried preparations and its preparation, which can solve the problems of large water content of sterile powder injections, slow dissolution speed of sterile powder injections, and phosphate creatine injections. Sodium acid is easy to decompose and other problems, to achieve the effect of improving solubility and stability, fast dissolution speed, safety and reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
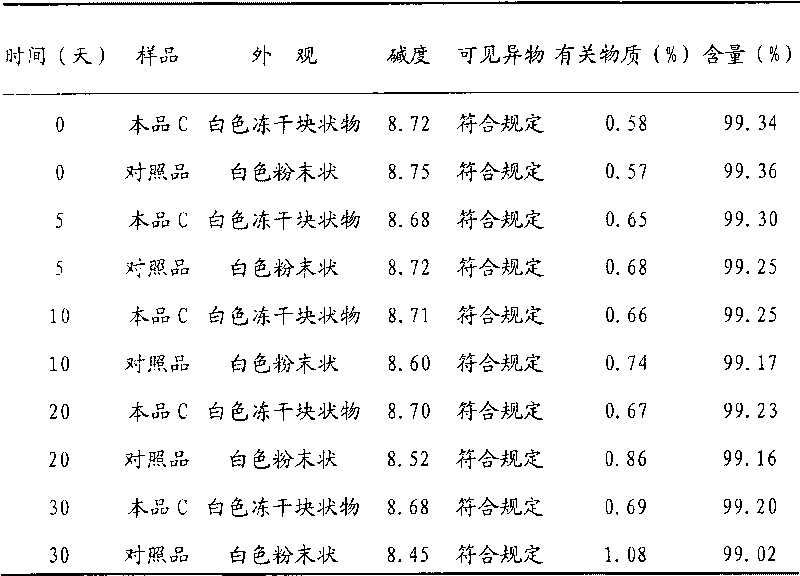
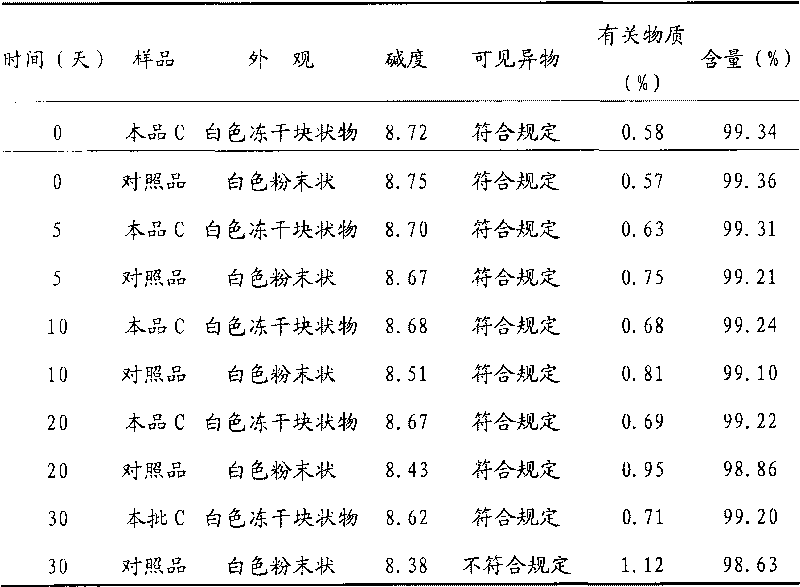
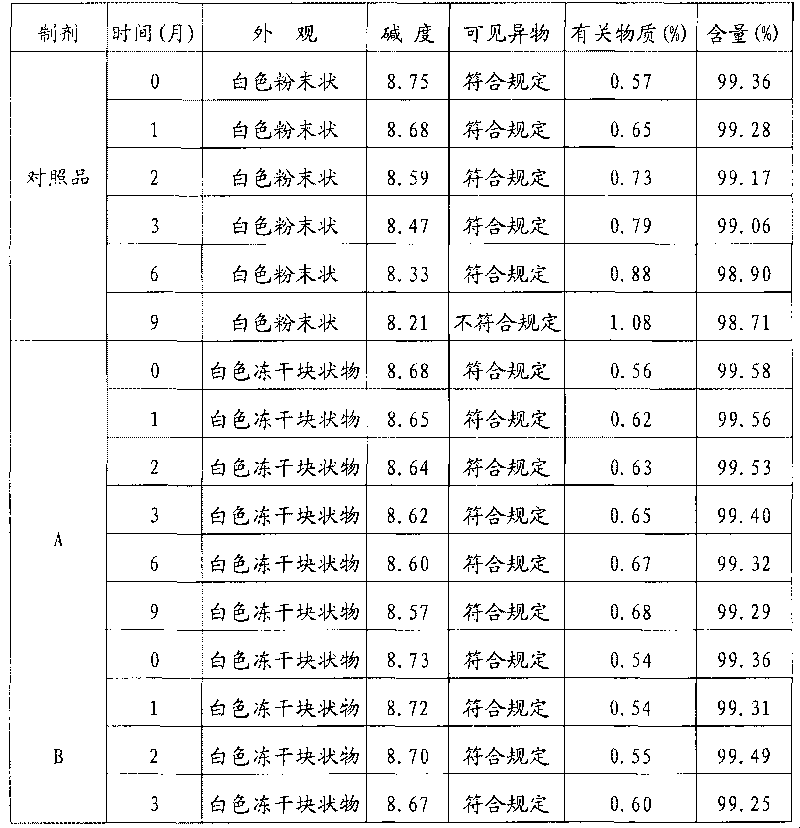
[0019] Take 500g of creatine phosphate sodium (manufactured by Beijing Lixiang Pharmaceutical Co., Ltd.) and 100g of mannitol (manufactured by Shandong Jiejing Group), add an appropriate amount of water for injection, and stir to dissolve completely. Add activated carbon according to the consumption of 0.05% (g / ml), stir at room temperature for 60 minutes, filter and decarbonize, and obtain the filtrate to sterilize with a 0.22 μm microporous filter element, after the filtrate is subpackaged, freeze-dry, seal, obtain phosphoric acid of the present invention Sodium creatine lyophilized formulation A.
Embodiment 2
[0021] Take 500 g of creatine phosphate sodium (manufactured by Beijing Lixiang Pharmaceutical Co., Ltd.) and 150 g of lactose, add an appropriate amount of water for injection, and stir to completely dissolve it. Add active carbon according to the consumption of 0.1% (g / ml), stir at room temperature for 60 minutes, filter and decarbonize, and the resulting filtrate is sterilized with a 0.22 μm microporous filter element, after the filtrate is subpackaged, freeze-dried and sealed to obtain the phosphoric acid of the present invention Sodium creatine freeze-dried formulation B.
Embodiment 3
[0023] Take 500g of creatine phosphate sodium (manufactured by Beijing Lixiang Pharmaceutical Co., Ltd.) and 200g of dextran (manufactured by Shandong Jinyang Pharmaceutical Co., Ltd.), add an appropriate amount of water for injection, and stir to dissolve completely. Add active carbon according to the consumption of 0.5% (g / ml), stir at 50 ℃ for 30 minutes, filter and decarbonize, and the obtained filtrate is sterilized with a 0.22 μm microporous filter element, after the filtrate is subpackaged, freeze-dried and sealed to obtain the Sodium creatine phosphate freeze-dried formulation C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com