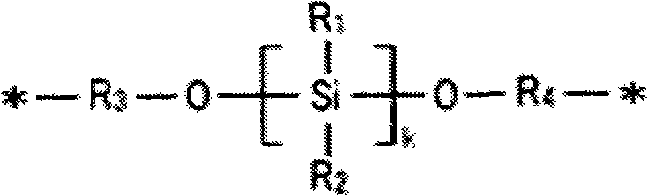
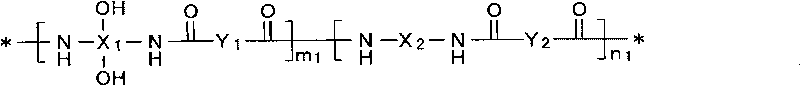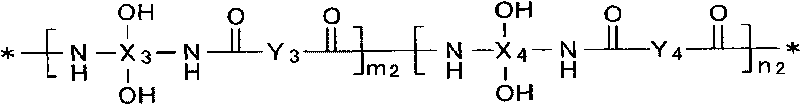Positive type photosensitive resin composition
一种光敏树脂、组合物的技术,应用在光学、光机械设备、仪器等方向,能够解决损害已固化薄膜机械性能、增大抑制层、材料不能被充分地显影等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0275] Synthesis Example 1: Synthesis of Polybenzoxazole Precursor (PBO-A)
[0276] In a four-necked flask equipped with a stirrer, a temperature control device, a nitrogen injector, and a condenser, 17.4 g of 2,2-bis(3-amino-4-hydroxyphenyl)-1,1,1,3 , 3,3-hexafluoropropane and 0.86 g of 1,3-bis(aminopropoxy)dimethylsiloxane were dissolved in 280 g of N-methyl-2-pyrrolidone (NMP) while nitrogen was in it pass. The above solution contained 9% by weight solids.
[0277] When the solid was completely dissolved, 9.9 g of pyridine was added to the solution. On the other hand, 13.3 g of 4,4'-oxydibenzoyl chloride was dissolved in 142 g of N-methyl-2-pyrrolidone (NMP). This solution was slowly added dropwise over 30 minutes while maintaining the former solution at a temperature of 0 to 5°C. Next, a reaction was performed at a temperature of 0 to 5° C. for 1 hour, and then the obtained solution was heated to room temperature and stirred for one hour.
[0278]Then, 1.6 g of 5-no...
Embodiment 2
[0281] Synthesis Example 2: Polybenzoxazole precursor (PBO-B 1 )Synthesis
[0282] In a four-necked flask equipped with a stirrer, a temperature control device, a nitrogen injector, and a condenser, 10.1 g of 2,2-bis(3-amino-4-hydroxyphenyl)-1,1,1,3 , 3,3-hexafluoropropane was dissolved in 111.1 g of N-methyl-2-pyrrolidone (NMP) while passing nitrogen gas therethrough. The solution contained 9 wt% solids.
[0283] To completely dissolve the solid, 4.2 g of pyridine was added to the solution. The obtained mixture was kept at a temperature of 0 to 5°C. On the other hand, 5.78 g of trans-3,6-endomethylene-1,2,3,6-tetrahydrophthaloyl chloride was dissolved in 100 g of N-methyl-2-pyrrolidone (NMP) . This solution was slowly added to the former solution in a dropwise manner over 30 minutes. The mixed solution was reacted at a temperature of 0 to 5° C. for 1 hour, then heated to room temperature and reacted for one hour.
[0284] Next, 1.2 g of 5-norbornene-2,3-dicarboxy...
Embodiment 3
[0288] Polybenzoxazole precursor (PBO-B 2 )Synthesis
[0289] In a four-necked flask equipped with a stirrer, a temperature control device, a nitrogen injector, and a condenser, 10.1 g of 2,2-bis(3-amino-4-hydroxyphenyl)-1,1,1,3 , 3,3-hexafluoropropane was dissolved in 111.1 g of N-methyl-2-pyrrolidone (NMP), while nitrogen gas was passed therethrough. The solution contained 9 wt% solids.
[0290] When the solid was completely dissolved, 4.2 g of pyridine was added to the solution. The resulting mixture was maintained at a temperature of 0 to 5°C. By dissolving 2.89g of trans-3,6-endomethylene-1,2,3,6-tetrahydrophthaloyl chloride and 3.9g of 4,4'-oxydibenzoyl chloride in 100g Another solution was prepared in N-methyl-2-pyrrolidone (NMP). This solution was added to the former solution in a dropwise manner over 30 minutes. The mixed solution was reacted at a temperature of 0 to 5° C. for 1 hour, then heated to room temperature and reacted for 1 hour.
[0291] Next, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight-average molecular weight | aaaaa | aaaaa |
| weight-average molecular weight | aaaaa | aaaaa |
| weight-average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com