Cordycepin extractive and application thereof in preparing hypoglycemic drugs or health-care foods
A health food and extract technology, applied in the field of biopharmaceuticals, can solve the problems of complex equipment, low extraction rate, and low yield, and achieve the effects of easy control of process conditions, hypoglycemic effect, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Preparation and parameter optimization of embodiment 1 cordycepin extract
[0021] The cordycepin extract of the present embodiment, its preparation method comprises the steps:
[0022] Firstly, the liquid culture solution of Cordyceps militaris obtained by conventional method was selected as the raw material, and the liquid culture solution of Cordyceps militaris was concentrated into 1 / 5 of the original volume through a rotary evaporator, and the concentration temperature was 60°C; the polysaccharide was precipitated with 4 times the volume of absolute ethanol for 24 hours; A low-speed large-capacity centrifuge was used to separate the precipitate, and the supernatant was collected as a column sample, in which the concentration of cordycepin was 16.09 μg / mL.
[0023] Different macroporous adsorption resins were selected: S-8, NKA-9, D-101 and AB-8 for adsorption and desorption. It was found that the static adsorption capacity of D-101 macroporous resin was the highes...
Embodiment 2
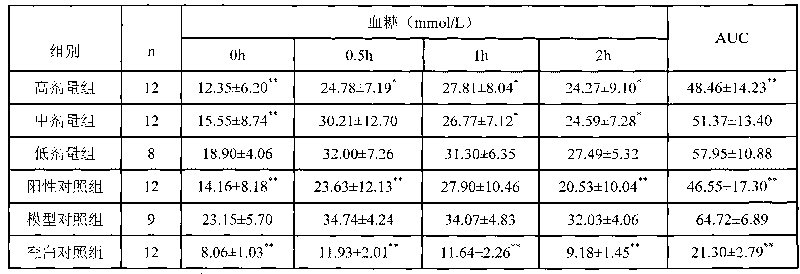
[0032] (1) Kunming SPF mice 20±2g, after three days of adaptive feeding, fasting without food and water for 14 hours, each mouse was intraperitoneally injected with 1% alloxan at a dose of 200 mg / kg once. After 72 hours, the tail was taken for venous blood to measure blood sugar, and fasted for 8 hours before taking blood. The animals with blood glucose concentration greater than 11.1mmol / l were selected as hyperglycemia model animals.
[0033] (2) The hyperglycemia model mice were divided into a hyperglycemia model control group, a positive drug control group and a high-dose cordycepin extract treatment group, with 12 mice in each group. Twelve normal mice were taken as blank control group. The cordycepin extract high-dose treatment group was injected intraperitoneally with 72 mg / kg d of the cordycepin extract prepared in Example 1, the positive drug control group was intraperitoneally injected with phenformin hydrochloride 70 g / kg d, the hyperglycemia model control group an...
Embodiment 3
[0036] (1) Kunming SPF mice 20 ± 2 g, after three days of adaptive feeding, fasting without food and water for 14 hours, each mouse was intraperitoneally injected with 1% alloxan at a dose of 200 mg / kg once. After 72 hours, the tail was taken for venous blood to measure blood sugar, and fasted for 8 hours before taking blood. The animals with blood glucose concentration greater than 11.1mmol / l were selected as hyperglycemia model animals.
[0037] (2) The hyperglycemia model mice were divided into a hyperglycemia model control group, a positive drug control group and a middle-dose cordycepin extract treatment group, with 12 mice in each group. Twelve normal mice were taken as blank control group. Cordycepin extract medium dose treatment group was injected intraperitoneally with 24 mg / kg d of cordycepin extract prepared in Example 1, the positive drug control group was intraperitoneally injected with phenformin hydrochloride 70 g / kg d, hyperglycemia model control group and bla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com