Process for precious metal recovery from a sulphide ore or concentrate or other feed material
A precious metal and sulfide technology, applied in the field of silver and platinum group metals to recover precious metals such as gold, can solve the problems of high cost, easy to cover recycled metals, and low recovery rate of gold and silver.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0261] The following examples show the results of the treatment of copper-gold concentrates using known techniques, namely pressure oxidation and leaching to extract copper, followed by cyanidation of the residue with appropriate amounts of cyanide at ambient pressure (in air) to extract gold and silver.
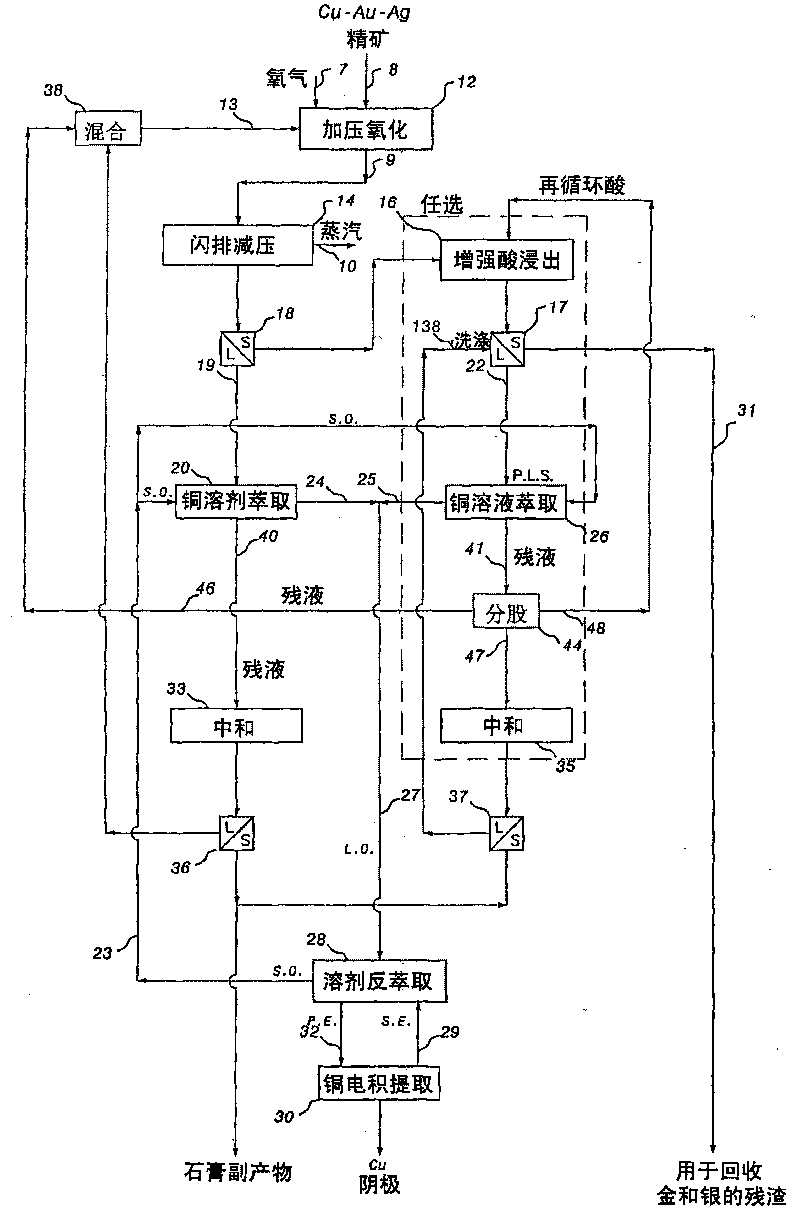
[0262] a) Extract copper
[0263] 143 g of Concentrate I, consisting mainly of chalcopyrite and 20% pyrite with a small amount of bornite, were wet ground in a laboratory rod mill such that 97% of the mineral particles passed through a 45 micron mesh screen. For copper concentrates, the higher pyrite content often results in excessive sulfur oxidation in hydrometallurgical copper recovery processes such as those described in the '708 patent. Use 1050mL containing 12g / L Cl, 12g / L Cu and 15g / L H 2 SO 4 CuSO 4 -CuCl 2 -H 2 SO 4 The solution slurries the finely ground solid with a solid density of 130 g / L. The slurry was placed in a 2L titanium autoclave, which was then ...
Embodiment 2
[0276] The following examples show the effect of increasing cyanide concentration on gold and silver recovery and on cyanide consumption. In order to minimize the loss of cyanide due to high concentration, NaCN was partially added at the beginning (10% of the total amount), and then the remaining 90% was added slowly throughout the reaction to compensate for the loss of cyanide and reduce the cyanide to cyanide. Compounds have always remained at a high level.
[0277] a) Extract copper
[0278] 143 g of Concentrate I (Table 1) were ground again and subjected to batch pressure oxidation under conditions similar to those described in Example 1, except that the residence time was reduced to 60 minutes. Gold and silver were extracted with washed residue containing 10.5g / t gold and 43g / t silver.
[0279] b) Extraction of gold and silver
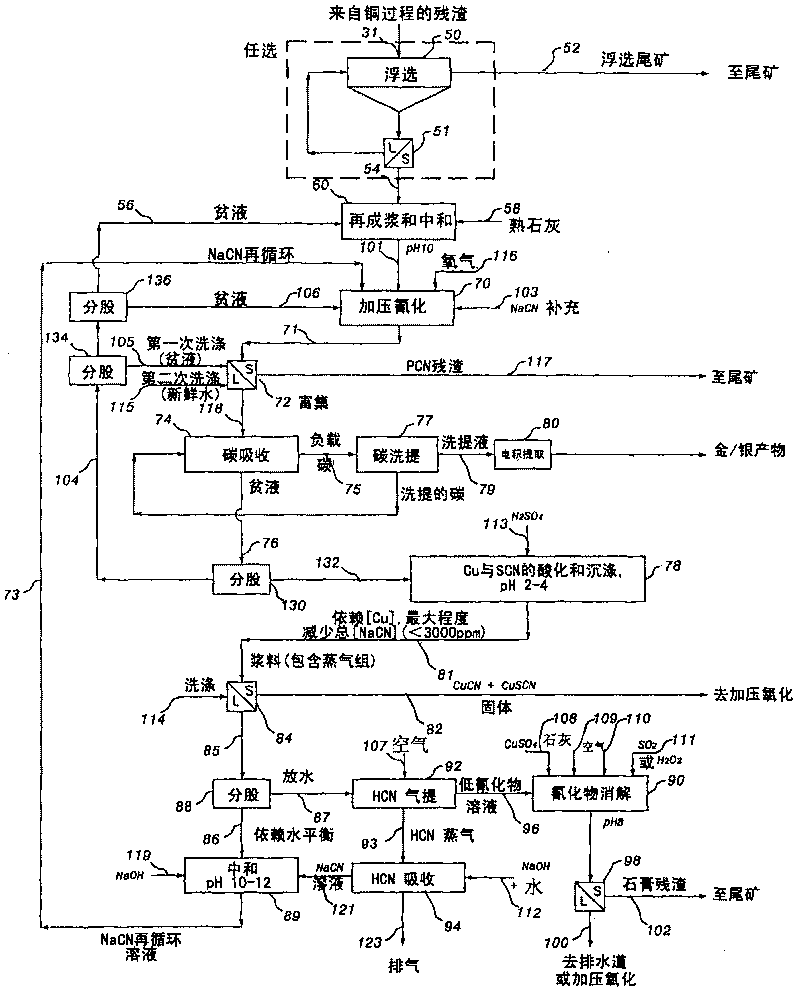
[0280] 121 g of the residue from the copper extraction step were reslurried with fresh water to a solid density of 400 g / L and then neutralize...
Embodiment 3
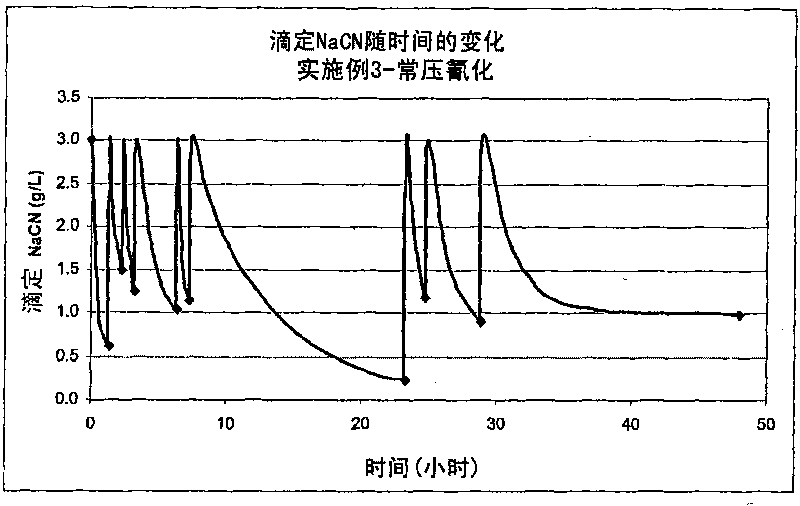
[0287] The following example replicates the conditions of Example 2, cyaniding at ambient air pressure with continuous addition of cyanide during leaching, but increasing the cyanide concentration even further to show its effect on the extraction of gold and silver.
[0288] a) Extract copper
[0289] Concentrate I was ground again and subjected to batch pressure oxidation under conditions similar to those described in Example 2.
[0290] After pressure oxidation under these conditions for 60 minutes, the slurry was cooled, filtered to produce a primary filtrate, which was then washed thoroughly with water to produce a residue containing 1.0% copper, 10.5g / t gold and 56g / t silver, and a secondary or wash filtrate. Based on the copper content in the residue, copper extraction was 97% with a mass loss of 17%.
[0291] b) Extraction of gold and silver
[0292] As in Examples 2 and 3, 75 g of the residue from the copper extraction step (on a dry weight basis) was reslurried in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com