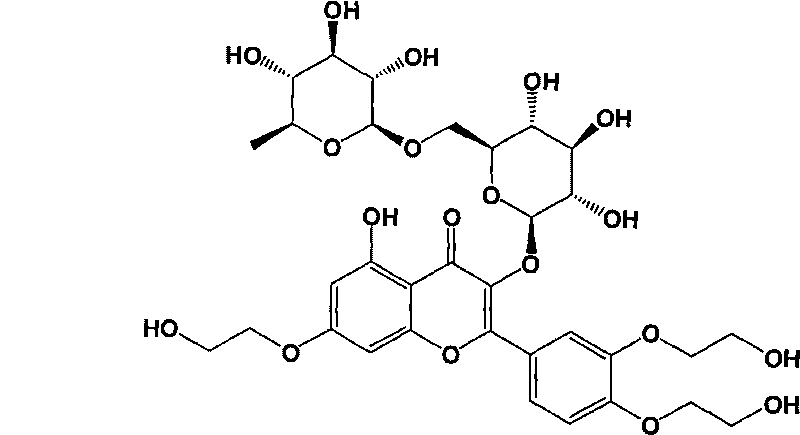
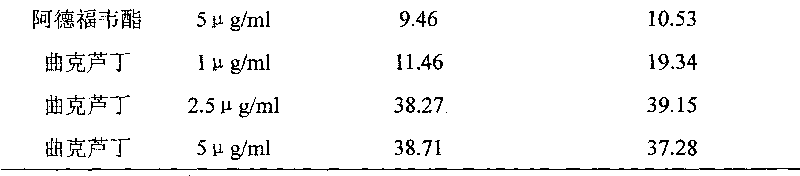Application of troxerutin in preparing medicine resisting viral hepatitis
A viral hepatitis and viral technology, applied in the direction of antiviral agents, pharmaceutical formulations, organic active ingredients, etc., can solve the problems of no drug treatment, lack of specific pathological animal models, etc., to increase selectivity and facilitate a wide range of The effect of easy use and availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 In vitro anti-hepatitis B virus test of troxerutin
[0022] 1. Materials and Methods
[0023] 1.1 Cell culture and drugs
[0024] Using HepG2 2.2.15 cells as a model, inoculate HepG2 2.2.15 cells to 25cm 2 In the culture flask, add 5ml of DMEM medium mixed culture solution (containing 10% FBS and 380μg / ml G418) to each bottle, place at 37°C, 5% CO 2 Passaging in a constant temperature incubator for 3 to 4 days. with 5% NaHCO 3 Adjust the pH to 7.2. (DMEM medium and fetal bovine serum are products of GIBCO; G418 is a product of Sigma).
[0025] Adefovir dipivoxil (product of Suzhou GlaxoSmithKline Pharmaceutical Co., Ltd.), dissolved in dimethyl sulfoxide to make a 1 mg / ml solution, was diluted to 5 μg / ml with culture medium.
[0026] Troxerutin (product of Shaanxi Senfu Biotechnology Co., Ltd.), dissolved in dimethyl sulfoxide to make a 1 mg / ml solution, was diluted with culture medium to 1 μg / ml, 2.5 μg / ml, and 5 μg / ml respectively. solution.
[0027]...
Embodiment 2
[0045] Example 2 In vivo anti-hepatitis B virus test of troxerutin
[0046] 1. Experimental materials:
[0047] 1.1 virus
[0048] Duck hepatitis B virus DNA (DHBV-DNA) strongly positive serum was collected from Shanghai shelduck and stored at -70°C.
[0049] 1.2 Animals
[0050] 1-day-old Peking duck (product of the animal farm of the Institute of Medicine and Plants, Beijing Academy of Medical Sciences).
[0051] 1.3 Drugs
[0052] Adefovir dipivoxil (product of Suzhou GlaxoSmithKline Pharmaceutical Co., Ltd., China); Troxerutin (product of Shaanxi Senfu Biotechnology Co., Ltd.). The above drugs were made into liposomes of 1 mg / ml respectively, diluted with water for injection and then administered.
[0053] 1.4 Reagent α-32 P-dCTP (product of Beijing Furui Company); gap translation kit (product of Promega Company); SephadexG-50, Ficoll PVP (product of Pharmacia, Sweden); SDS (product of Merck, Germany); Protist DNA, bovine serum albumin (product of Institute of Biophy...
Embodiment 3
[0070] Example 3 Experiment of the hepatoprotective effect of troxerutin on the immune liver injury model in mice induced by bacillus Calmette-Guerin (BCG) and lipopolysaccharide (LPS)
[0071] 1. Experimental materials
[0072] 1.1 Experimental animals
[0073] Kunming mice, ♂, 18-22g (product of Experimental Animal Center of Second Military Medical University).
[0074] 1.2 Drugs and reagents
[0075] Troxerutin (product of Shanxi Senfu Biotechnology Co., Ltd.) was made into 1 mg / ml liposome, diluted with water and administered by injection.
[0076] Ribavirin injection (product of Shanghai Xinyi Pharmaceutical Co., Ltd.).
[0077] Bacillus Calmette-Guerin (BCG) (product of Shanghai Biological Products Company).
[0078] Lipopolysaccharide (LPS) (product of Sigma).
[0079] ALT, AST kit (product of Nanjing Jiancheng Institute of Bioengineering)
[0080] 2. Experimental methods and results
[0081] Animals were divided into random groups, tail ivBCG2Sit / only, the norma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com