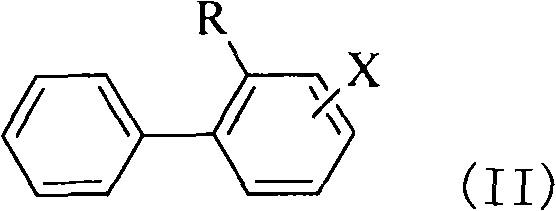Preparation method of hydroxymethyl substitutent o-alkyl biphenyl and intermediate thereof
A technology of alkyl biphenyl and hydroxymethyl, applied in the field of preparation of hydroxymethyl substituted o-alkyl biphenyl and its intermediates, can solve the problems of long route, high production cost, lack of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0098] The preparation method of halogenated o-alkyl biphenyl intermediate of the present invention comprises the steps:
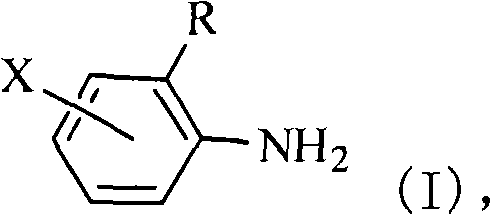
[0099] (a) providing the halogenated o-alkylaniline shown in formula (I) as raw material:
[0100]
[0101] where R is C n h 2n+1 , n=1, 2, 3, 4, or 5;
[0102] X is F, Cl, Br, or I;
[0103] (b) In the presence of metal catalysts, acid cocatalysts and nitrite esters, the halogenated o-alkylanilines of the formula (I) of step (a) carry out diazo coupling reactions with benzene to obtain formula (II) ) of halogenated o-alkylbiphenyl compounds:
[0104]
[0105] Wherein R and X in formula (II) have the same meanings as formula (I).
[0106] In the above diazo coupling reaction, the amino group in the compound of formula (I) is coupled by diazo to obtain the compound of formula (II).
[0107] In a preferred example, the halogenated o-alkylaniline of the formula (I) is 3-chloro-2-methylaniline; the halogenated o-alkylbiphenyl compound of the formul...
Embodiment 1
[0152] Put 3-chloro-2-methylaniline (71g, 0.5mol) and benzene (300ml, 3.38mol) into a 1000ml four-necked flask, and start stirring. Ferric chloride (2g, 0.012mol), triethyl orthoformate Ester (55g, 0.34mol) was put into the reactor. The oil bath controlled the reaction temperature at 40°C, and isopropyl nitrite (80g, 0.9mol) and benzene (200mL, 2.25mol) were added to the constant pressure dropping funnel , under the condition of maintaining a constant reaction temperature of 40°C, drop it into the reactor. After the dropwise addition of isopropyl nitrite, stir at 40°C for 2 hours. Cool down to room temperature, drop in urea (50g) and 30% hydrochloric acid (200g) After phase separation, the organic layer was rectified. Recover benzene first, and then continue rectifying to obtain the refined product 3-chloro-2-methylbiphenyl (76g, 0.37mol). The gas chromatography analysis purity is 99.5%, and the yield is 75%.
Embodiment 2~ Embodiment 9
[0154] Metal catalyst consumption or kind are changed, and other is with embodiment 1, and its result is shown in Table 1
[0155] Table 1
[0156] implement
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com