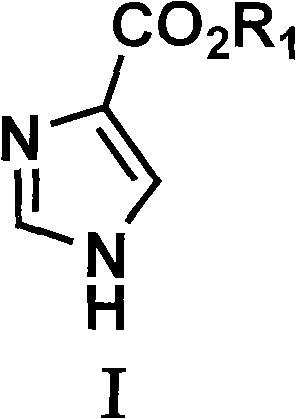
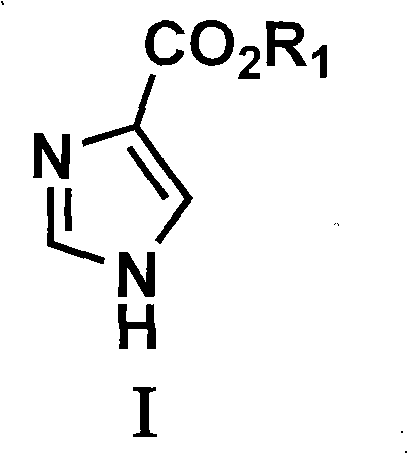
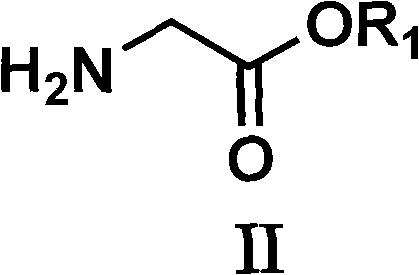
Preparation method of glyoxaline formic ether and derivative thereof
A derivative, imidazole technology, applied in the field of pharmaceutical intermediate synthesis, can solve the problems of difficult purification of products, many side reactions, difficult industrial application and the like, and achieves the effects of wide and convenient sources, low production cost and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 imidazole-4-formic acid ester
[0041] 1) Preparation of glycine ethyl ester
[0042] In glycine (75 grams, 1 mole) and ethanol (450 milliliters), then add catalytic amount of sodium bisulfate, heat, vigorously reflux and stir for 2 to 5 hours; the reaction mixture is cooled to room temperature, suction filtered, and the organic solvent is removed, 98 g (95%) of the desired product were obtained.
[0043] 2) Preparation of N-acetylated glycine ethyl ester
[0044] Glycine ethyl ester (103 g, 1 mole) was dissolved in 600 ml of ethyl acetate, a catalytic amount of DMAP was added, heated, vigorously refluxed and stirred for 2 to 5 hours; the reaction mixture was cooled to room temperature, washed with saturated brine several times, Dry over anhydrous sodium sulfate and remove the organic solvent to obtain 135 g (93%) of the target product.
[0045] 3) Preparation of mercapto-substituted imidazole carboxylic acid compounds
[0046]Sodium ...
Embodiment 2
[0050] The preparation of embodiment 2 imidazole-4-carboxylic acid
[0051] 1) Preparation of glycine methyl ester
[0052] In glycine (75 grams, 1 mole) and methanol (500 milliliters), then pass through hydrogen chloride gas to saturation, heat, and vigorously reflux and stir for 2 to 4 hours; the reaction mixture is cooled to room temperature, suction filtered, and the organic solvent is removed to obtain The target product was 86 grams.
[0053] 2) Preparation of N-acetylated glycine methyl ester
[0054] Glycine methyl ester (89 g, 1 mole) was dissolved in 600 ml of methyl acetate, a catalytic amount of triethylamine was added, heated, and vigorously refluxed and stirred for 2 to 5 hours; the reaction mixture was cooled to room temperature, washed with saturated brine After drying with anhydrous sodium sulfate, the organic solvent was removed to obtain 111 grams of the target product.
[0055] 3) Preparation of mercapto-substituted imidazole carboxylic acid compounds
...
Embodiment 3
[0064] The preparation of embodiment 3 4-hydroxymethylimidazole
[0065] 1) Preparation of glycine ethyl ester
[0066] In glycine (75 g, 1 mole) and ethanol (450 ml), then add a catalytic amount of thionyl chloride, heat, and vigorously reflux and stir for 2 to 5 hours; cool the reaction mixture to room temperature, suction filter, and remove the organic solvent , to obtain 97 g of the target product.
[0067] 2) Preparation of N-acetylated glycine ethyl ester
[0068] Glycine ethyl ester (103 g, 1 mole) was dissolved in 600 ml of ethyl acetate, a catalytic amount of pyridine was added, heated, and vigorously refluxed and stirred for 2 to 5 hours; the reaction mixture was cooled to room temperature, washed with saturated brine several times, Dry over anhydrous sodium sulfate and remove the organic solvent to obtain 120 g (90%) of the target product.
[0069] 3) Preparation of mercapto-substituted imidazole carboxylic acid compound
[0070] Sodium ethylate (102 grams, 1.5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com