Process for preparing bepotastine and intermediates used therein
A technology of bepotastine and intermediates, applied in the preparation of bepotastine and the field of intermediates used therein, can solve problems such as not being effective
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
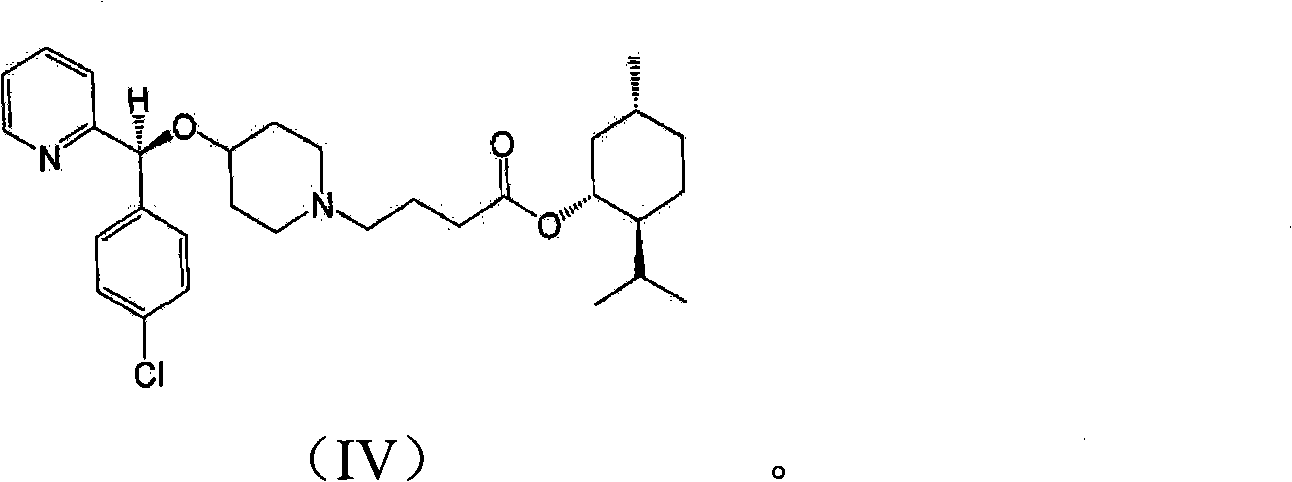
[0031] Step 5) preparation of bepotastine
[0032] In reaction step 5), bepotastine l-menthyl ester of formula (IV) is hydrolyzed in the presence of a base to produce bepotastine.
[0033] Sodium hydroxide, potassium hydroxide, or the like can be used in an amount of 1 to 5 equivalents based on bepotastine l-menthyl ester.
[0034] This hydrolysis reaction is carried out in a mixture of water and an organic solvent selected from the group consisting of methanol, ethanol, isopropanol, acetone, acetonitrile and tetrahydrofuran at a temperature between 10°C and 60°C. Preferably, the mixing ratio of water and organic solvent is 1:0.05-1:20.
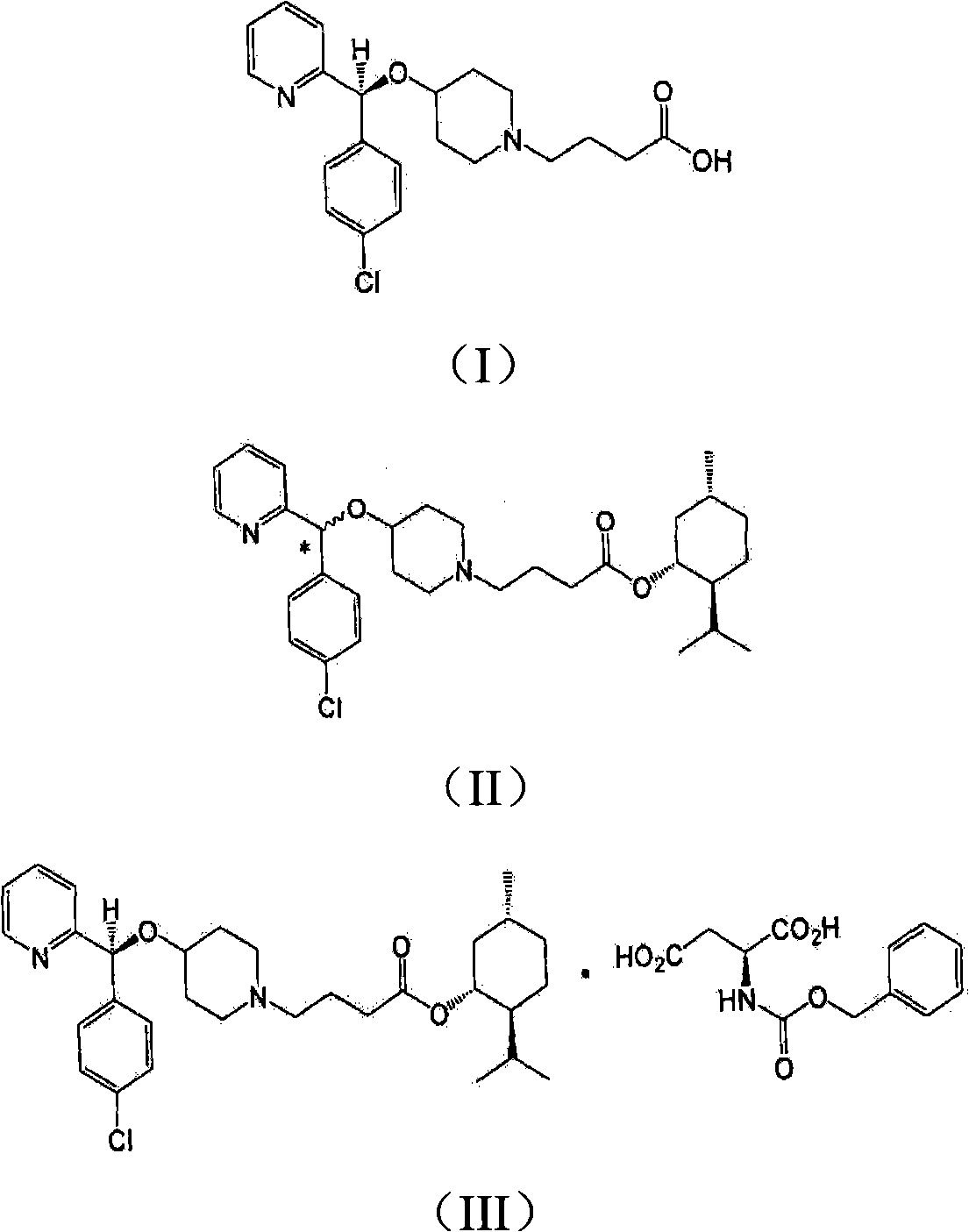
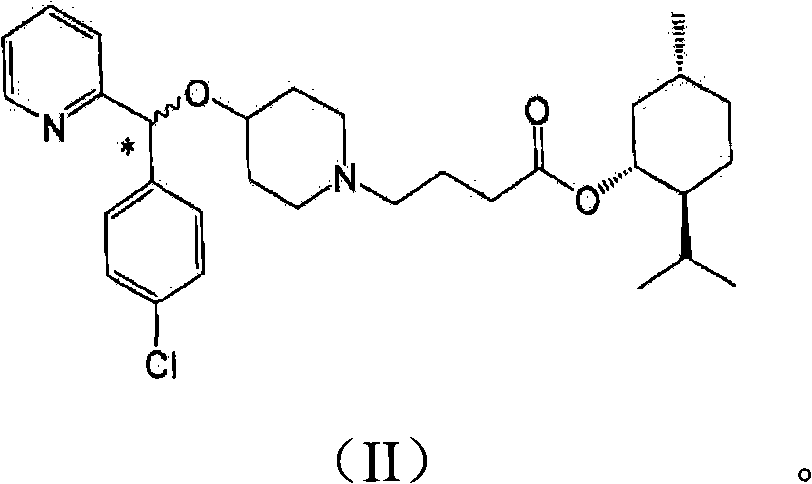
[0035] In addition, the present invention may further comprise the following step: after filtering the bepotastine l-menthyl ester N-benzyloxycarbonyl L-aspartic acid of the formula (III) precipitated in the reaction step 2), recovering rich Bepotastine l-menthyl ester containing (R)-isomer and treatment of recovered material with acid to...
preparation example 1
[0059] Preparation Example 1: Preparation of l-menthyl 4-bromobutyrate
[0060] 14.6g of 1-menthol and 14.8ml of pyridine were dissolved in 150ml of dichloromethane, and the solution obtained by dissolving 17.0g of 4-bromobutyryl chloride in 20ml of dichloromethane was slowly added dropwise to it, and the resulting mixture was cooled to room temperature. Stir for 1 hour. The reaction mixture was washed with 100 ml of water and the solvent was removed under reduced pressure to afford 27 g (97%) of the title compound as an oil.
[0061] 1 H-NMR (DMSO-d 6 , ppm): δ4.7(m, 1H), 3.5(t, 2H), 2.5(t, 2H), 2.2(m, 2H), 2.0(m, 1H), 1.9(m, 1H), 1.7( m, 2H), 1.5 (m, 1H), 1.3 (m, 1H), 1.1 (m, 3H), 0.9 (d, 6H), 0.7 (d, 3H).
[0062] IR (KBr, cm -1 ): 2956, 2928, 2870, 1729, 1456, 1370, 1251, 1205, 1177, 1129, 984.
preparation example 2
[0063] Preparation Example 2: Preparation of l-menthyl 4-chlorobutyrate
[0064] 1.0g of 1-menthol and 1.0ml of pyridine were dissolved in 5.0ml of dichloromethane, and the solution obtained by dissolving 0.7ml of 4-chlorobutyryl chloride in 5.0ml of dichloromethane was slowly added dropwise to it, and the resulting The mixture was stirred at room temperature for 1 hour. The reaction mixture was washed with 20 ml of water, and the solvent was removed under reduced pressure to obtain 1.6 g (99%) of the title compound as an oil.
[0065] 1 H-NMR (DMSO-d 6 , ppm): δ4.7(m, 1H), 3.6(t, 2H), 2.5(t, 2H), 2.1(m, 2H), 2.0(m, 1H), 1.9(m, 1H), 1.7( m, 2H), 1.5(m, 1H), 1.4(m, 1H), 1.2(m, 3H), 0.9(d, 6H), 0.8(d, 3H).
[0066] IR (KBr, cm -1 ): 2956, 2929, 2869, 1729, 1456, 1386, 1371, 1308, 1204, 1177, 1010, 984, 964, 913.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com