Radiation sensitizer used for brain tumor
A technology of radiation and sensitizers, applied in the directions of antitumor drugs, medical preparations containing active ingredients, organic active ingredients, etc., can solve problems such as undeveloped pharmaceuticals, and achieve the effect of improving the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example
[0029] The radiation sensitizer of the present invention is made according to the following Tables 1 and 2. That is, the formulation components are heated, melted, sterilized and filtered, and returned to room temperature to prepare an injection.
[0030] Table 1
[0031]
[0032] Table 2
[0033]
[0034] These radiation sensitizers were left at 20°C. As a result, no crystals were precipitated until 5 hours, and crystals were observed after 22 hours. The degree of radiation sensitizer 4>radiation sensitizer 1> The order of radiation sensitizer 2>radiation sensitizer 3. Thus, addition of creatinine was judged to increase solubility. In view of the metabolic rate of doradazole, such an effect is beneficial to improve the pharmacokinetic properties.
Embodiment 1
[0038]
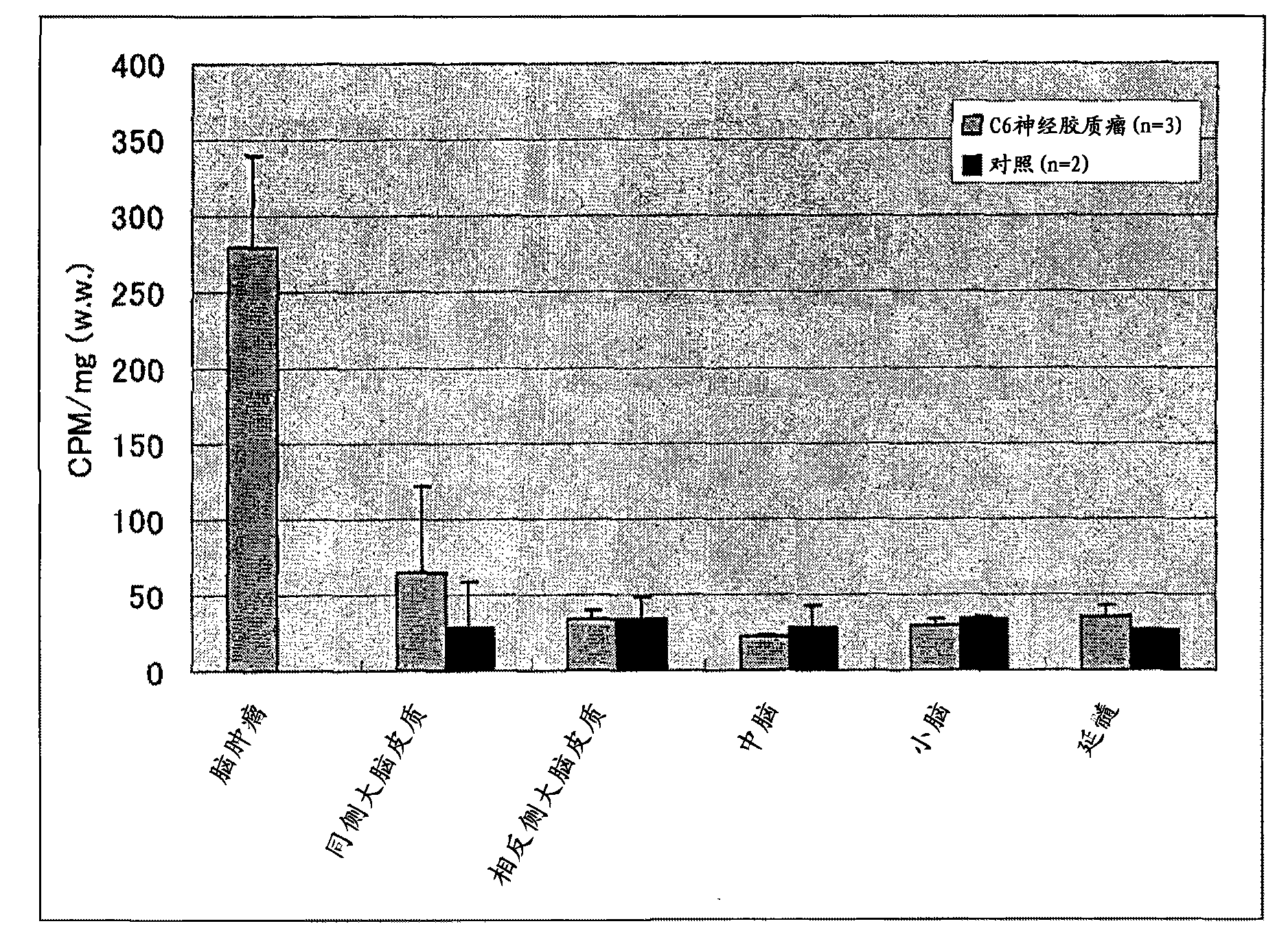
[0039] Male 11-week-old Fisher344 rats (Fisher344) were used as animals, and 1×10 6 C6 glioma cells were inoculated into the right caudate nucleus, and the growth of the tumor was confirmed by MRI at 1 week and 2 weeks after inoculation, and at 15 days 14 Cdoladazole (PR350) was dissolved in 3 mg / Kg normal saline and administered from the right common carotid artery. After 30 minutes, it was euthanized, and the brain was removed and divided into tumor part, ipsilateral cerebral cortex, opposite cerebral cortex, and middle cerebral cortex. Brain, cerebellum and medulla oblongata. After the wet weight was determined, the fractions were chopped into 150 mg and dissolved in Solibable 1 mL. Add 0.5 mL of hydrogen peroxide heated at 60°C for 3 hours, heat at 60°C for 1 hour, add 10 mL of liquid scintillation cocktail, and count with a liquid scintillation counter 14 CPM for Fraction C. The results are shown in figure 1 . The control example was only given normal sali...
Embodiment 2
[0041]
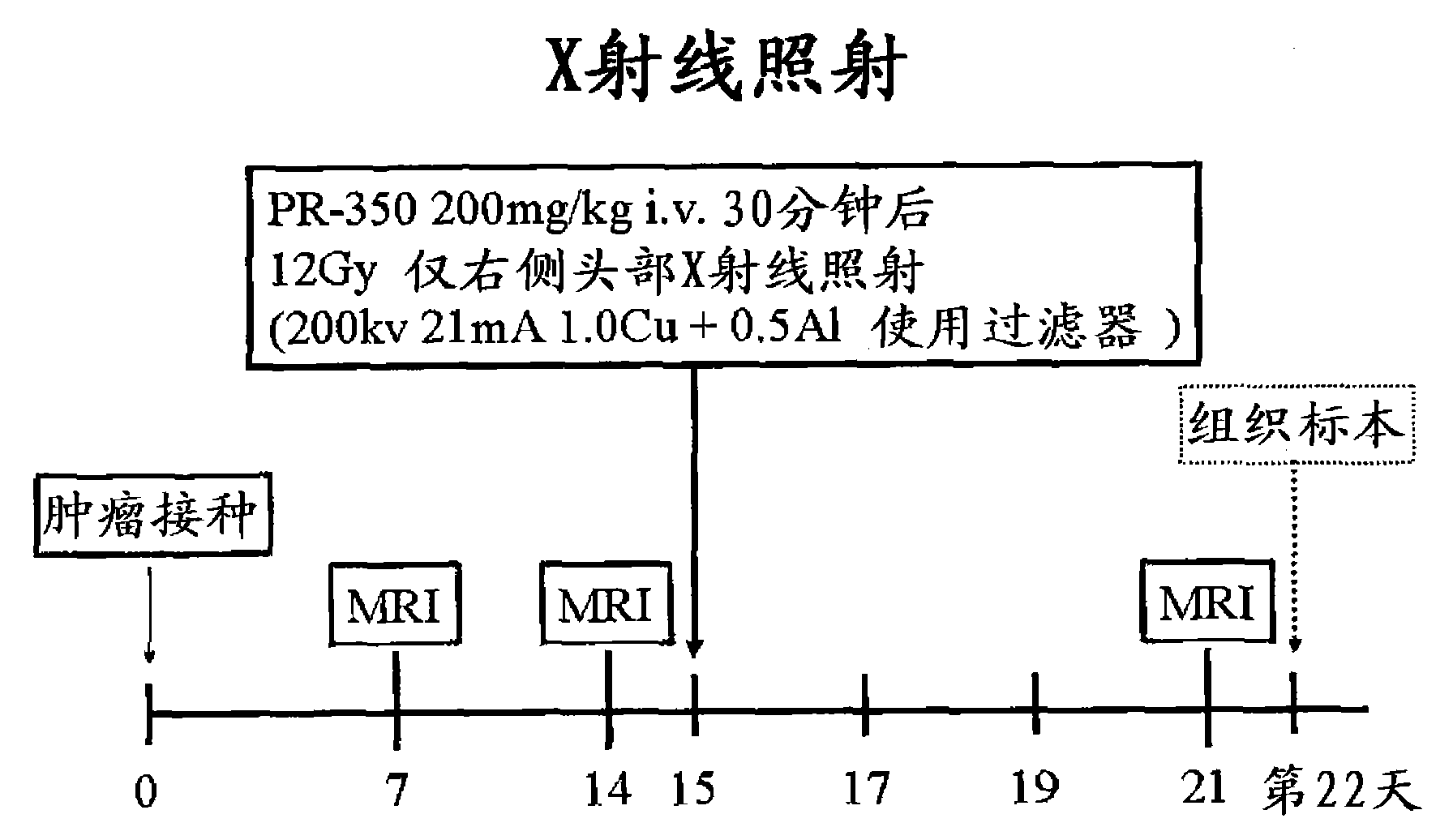
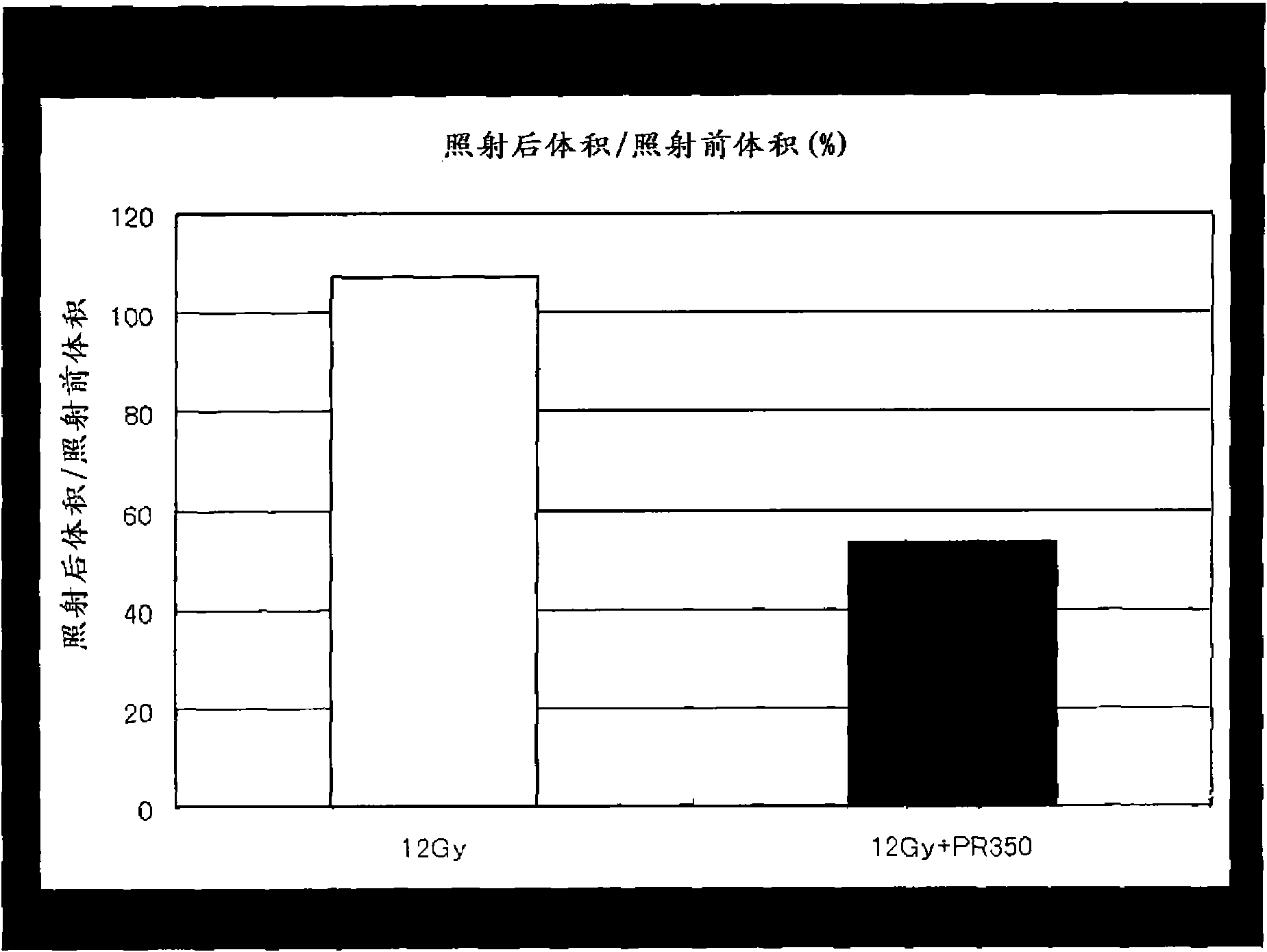
[0042] according to figure 2 In the sequence shown, adopt the method of Example 1 to inoculate C6 glioma cells, confirm the growth of the tumor with MRI in 1 week and 2 weeks after inoculation, and dissolve doradazole (PR350) in 3 mg / Kg at 15 days Physiological saline was administered from the right common carotid artery, and the brain tumor (right head) was irradiated with 12Gy of radiation immediately, and the feeding was continued. On the 21st day, the size of the tumor was measured again by MRI. The control group was given normal saline only instead of the normal saline solution of doradazole. Radiation exposure was performed in both groups. The tumor sizes on day 21 of the doradazole-administered group and the control group are shown in image 3 . It was judged that the tumor shrank by a significant difference due to the radiosensitizing effect of doradazole.
[0043] The present invention can be used as a radiation sensitizer for brain tumors.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com