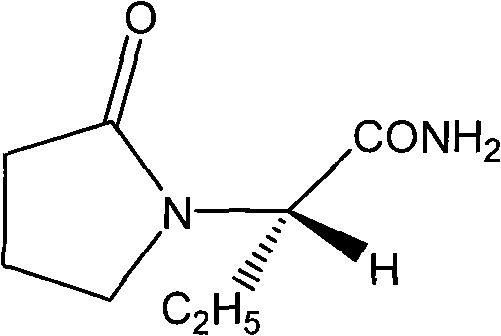
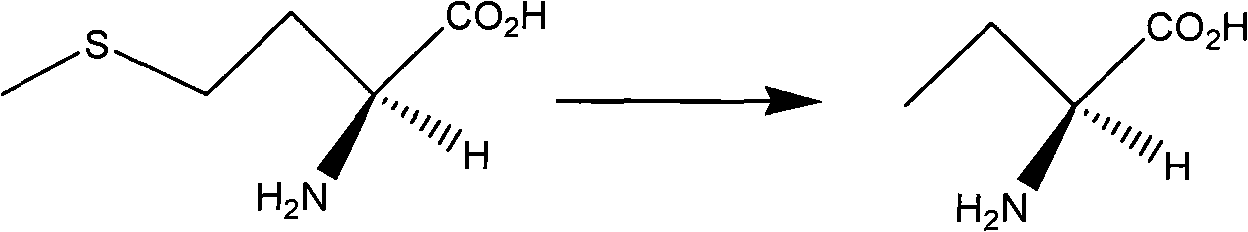Method for preparing levetiracetam
A technology for methionine and products, applied in the direction of organic chemistry, etc., can solve the problems of reduced yield and suboptimal synthesis process, and achieves the effects of simple operation, easy availability of raw materials, and simple operation steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The first step: the synthesis of (S)-ethylglycine
[0024] Dissolve L-methionine (50 g, 336 mmoL) in 4 L of distilled water, then add 500 g of T-1 Raney nickel, stir vigorously and heat. React at 75-80°C for 4h, cool down and filter to remove the catalyst. The filtrate was treated with 1% 8-hydroxyquinoline in CHCl 3 Liquid extraction (2×1000mL), then CHCl 3 Wash (3 x 1200 mL). The washed aqueous phase was concentrated and evaporated to obtain 32.0 g of the product, the purity of which was detected by normalization by gas chromatography was 98.9%, the yield was 95.0%, and the melting range was 273-275°C.
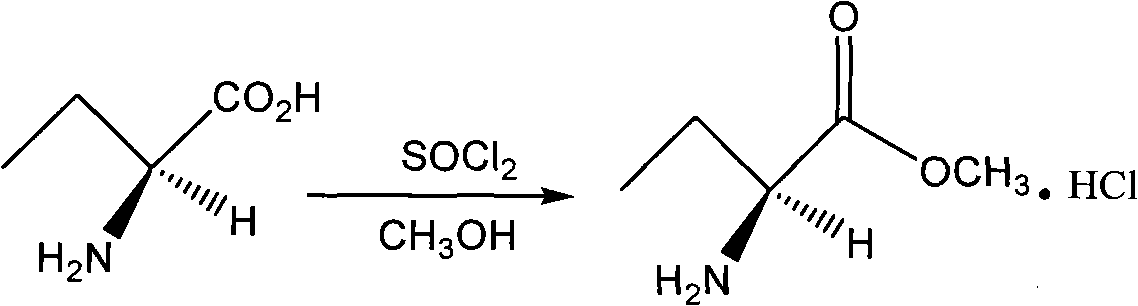
[0025] The second step: the synthesis of (S)-ethylglycine methyl ester hydrochloride
[0026] (S)-Ethylglycine (25.76g, 230mmol) was added to 150mL of anhydrous methanol, stirred, and 37.0mL of thionyl chloride was slowly added dropwise at -10°C, after the drop was completed, the temperature was naturally raised to room temperature and stirred for 5h , the solven...
Embodiment 2
[0033] Except that the first step is different from the first step of embodiment 1, others are the same, and the following embodiments are the same.
[0034] Operate in the same way as in the first step of Example 1, only change the T-1 Raney nickel 500g used therein to 300g of W-2 Raney nickel, and keep the others unchanged to obtain (S)-ethylglycine 31.5g, which is normalized by gas chromatography The purity detected by Yihua is 99.0%, the yield is 94.8%, and the melting range is 273-275°C.
[0035] Total yield based on L-methionine: 47.8%.
Embodiment 3
[0037] Operate in the same way as in the first step of Example 1, only change the T-1 Raney nickel 500g used therein to 320g of W-2 Raney nickel, and the others remain unchanged to obtain (S)-ethylglycine 32.0g, and the gas chromatography normalized The purity detected by Yihua is 99.0%, the yield is about 95.0%, and the melting range: 273-275°C.
[0038] The total yield in terms of L-methionine is about: 48.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com