Method for preparing high-purity superfine zinc sulfide
A zinc sulfide, high-purity technology, applied in the direction of zinc sulfide, can solve the problems of high equipment cost, complicated process control, and no practical application examples, and achieve the effects of production cost control, environmental pollution avoidance, and zero pollution discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
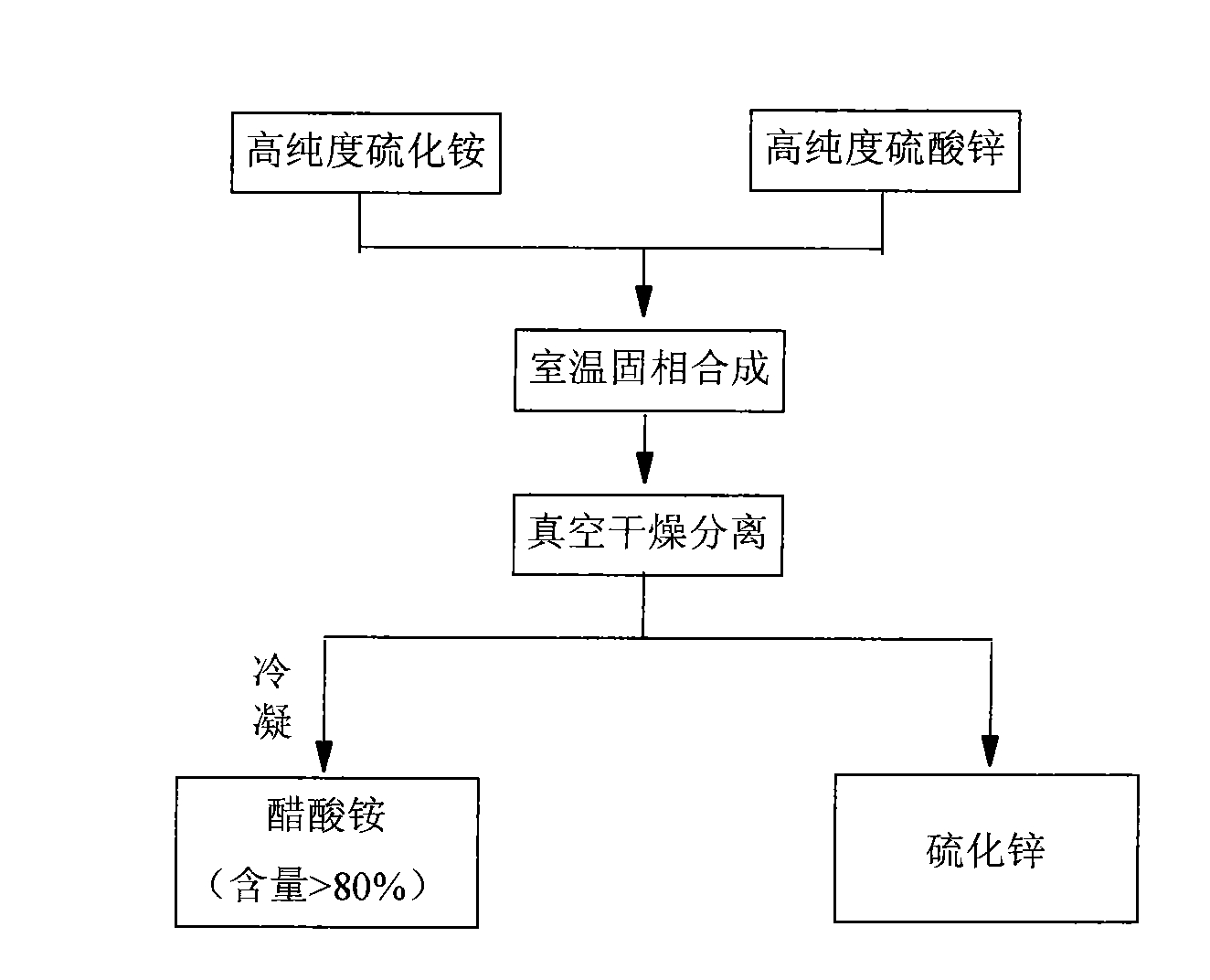
[0019] Embodiment 1: Get 40kg content and be that 99.90% zinc acetate and 5kg content are 99.90% ammonium sulfide in solid phase synthesis at room temperature, synthesis pressure-0.1~0.1MPa, time 1-24 hours, PH in the synthesis system is 4- 8. After the reaction is completed, vacuum-dry at -0.1-0.1MPa, 0-70°C, and recover after condensation of ammonium acetate (content > 80%). The dried product is zinc sulfide with a particle size of 50-200nm. Rate 97%-100%.
Embodiment 2
[0020] Example 2: 30kg of zinc formate with a content of 99.00% and 8kg of ammonium sulfide with a content of 99.00% are synthesized in solid phase at room temperature, the synthesis pressure is -0.1 to 0.1MPa, the time is 1-24 hours, and the pH in the synthesis system is 4- 8. After the reaction is completed, vacuum-dry at -0.1-0.1MPa, 0-70°C, and recover after condensation of ammonium formate (content > 80%). The dried product is zinc sulfide with a particle size of 50-200nm. Rate 98%-100%.
Embodiment 3
[0021] Example 3: 35kg of zinc chloride with a content of 99.50% and 10kg of ammonium sulfide with a content of 99.50% are synthesized in solid phase at room temperature, the synthesis pressure is -0.1 to 0.1 MPa, the time is 1-24 hours, and the pH in the synthesis system is 4 -8. After the reaction is completed, vacuum-dry at -0.1-0.1MPa, 0-70°C, condense ammonium chloride (content > 80%) and recover, and the dried product is zinc sulfide with a particle size between 50-200nm , Yield 95%-100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com