Nano-zinc base desulfurizing agent selecting oxidized sulfur compound as elemental sulfur and preparation method thereof
A technology of selective oxidation and base desulfurization agent, applied in chemical instruments and methods, separation methods, separation of dispersed particles, etc., can solve the problems of zinc oxide desulfurization ability reduction, high precision of zinc oxide desulfurization, desulfurization agent sintering, etc., to achieve sulfur capacity Increase, increase sulfur capacity, increase the effect of sulfur capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
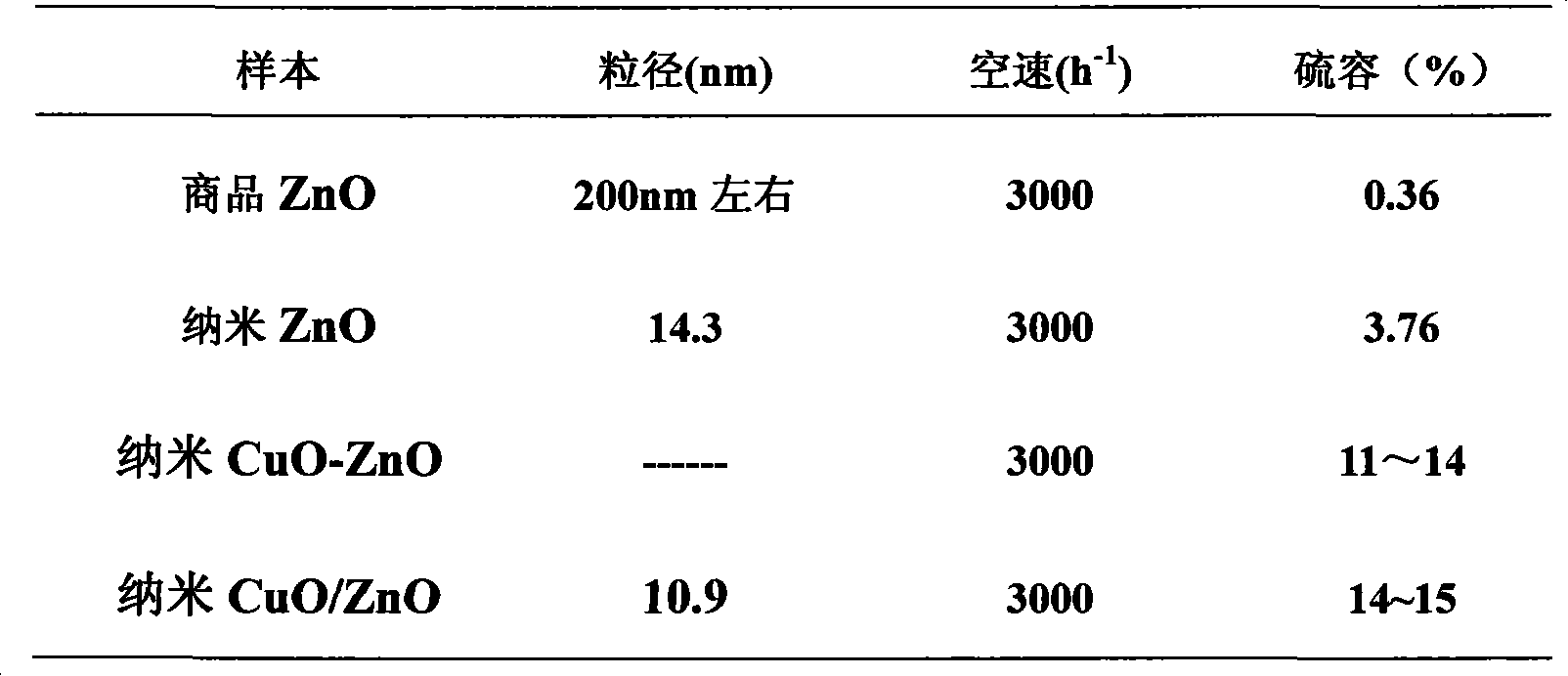
[0019] The nanometer CuO / ZnO desulfurizer has the best desulfurization performance when the mass fraction ratio of CuO and ZnO is 1:18.63.
[0020] Preparation of nano CuO / ZnO desulfurizer:
[0021] Copper nitrate (Cu(NO 3 ) 2 ·3H 2 O) and zinc nitrate (Zn(NO 3 ) 2 ·6H 2 O) The ratio of parts by mass is 1:22.26, urea and two kinds of nitrates (Cu(NO 3 ) 2 ·3H 2 O and Zn(NO 3 ) 2 ·6H 2 O) the total mass fraction ratio is a ratio of 1: 4.03, Zn(NO 3 ) 2 ·6H 2 O, Cu(NO 3 ) 2 ·3H 2 O and urea (CO(NH 2 ) 2 ) were dissolved in water respectively, filtered and mixed, the mixture was put into a high-pressure reactor, heated to 110°C for 1 hour, and the reaction mixture was filtered, washed, and dried at 120°C for 2 hours to obtain a precursor. ℃ roasting for 2 hours to prepare nano CuO / ZnO desulfurizer.
Embodiment 2
[0023] The nanometer zinc-based desulfurizer CuO / ZnO, which selectively oxidizes sulfur-containing compounds to elemental sulfur, consists of copper oxide (CuO) and zinc oxide (ZnO), with a mass-number ratio of 1:6.07. It is characterized in that: copper nitrate (Cu(NO 3 ) 2 ·3H 2 O) and zinc nitrate (Zn(NO 3 ) 2 ·6H 2 O) as the raw material, urea as the precipitant, that is, copper nitrate (Cu(NO 3 ) 2 ·3H 2 O) and zinc nitrate (Zn(NO 3 ) 2 ·6H 2 O) The ratio of parts by mass is 1:7.26, urea and two kinds of nitrates (Cu(NO 3 ) 2 ·3H 2 O and Zn(NO 3 ) 2 ·6H 2 O) the total mass fraction ratio is a ratio of 1: 4.03, Zn(NO 3 ) 2 ·6H 2 O, Cu(NO 3 ) 2 ·3H 2 O and urea (CO(NH 2 ) 2 ) were dissolved in water respectively, filtered and mixed, the mixture was put into a high-pressure reactor, heated to 110°C for 1 hour, and the reaction mixture was filtered, washed, and dried at 120°C for 2 hours to obtain a precursor. ℃ roasting for 2 hours to prepare nano CuO...
Embodiment 3
[0025] The composition includes copper oxide (CuO) and zinc oxide (ZnO), and the ratio of parts by mass is 1:50.62. It is characterized in that: copper nitrate (Cu(NO 3 ) 2 ·3H 2 O) and zinc nitrate (Zn(NO 3 ) 2 ·6H 2 O) as the raw material, urea as the precipitant, that is, copper nitrate (Cu(NO 3 ) 2 ·3H 2 O) and zinc nitrate (Zn(NO 3 ) 2 ·6H 2 O) The ratio of parts by mass is 1:60.50, urea and two kinds of nitrates (Cu(NO 3 ) 2 ·3H 2 O and Zn(NO 3 ) 2 ·6H 2 O) the total mass fraction ratio is a ratio of 1: 4.03, Zn(NO 3 ) 2 ·6H 2 O, Cu(NO 3 ) 2 ·3H 2 O and urea (CO(NH 2 ) 2) were dissolved in water respectively, filtered and mixed, the mixture was put into a high-pressure reactor, heated to 110°C for 1 hour, and the reaction mixture was filtered, washed, and dried at 120°C for 2 hours to obtain a precursor. ℃ roasting for 2 hours to prepare nano CuO / ZnO desulfurizer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com