High affinity combined sites of hgfr and method for identifying its antagonist
A domain, hepatocyte growth factor technology, applied in the field of hepatocyte growth factor receptor protein, can solve problems such as hindering the separation of therapeutic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] The invention will be described in detail in some related preferred embodiments by way of non-limiting examples with reference to the accompanying drawings, in which:
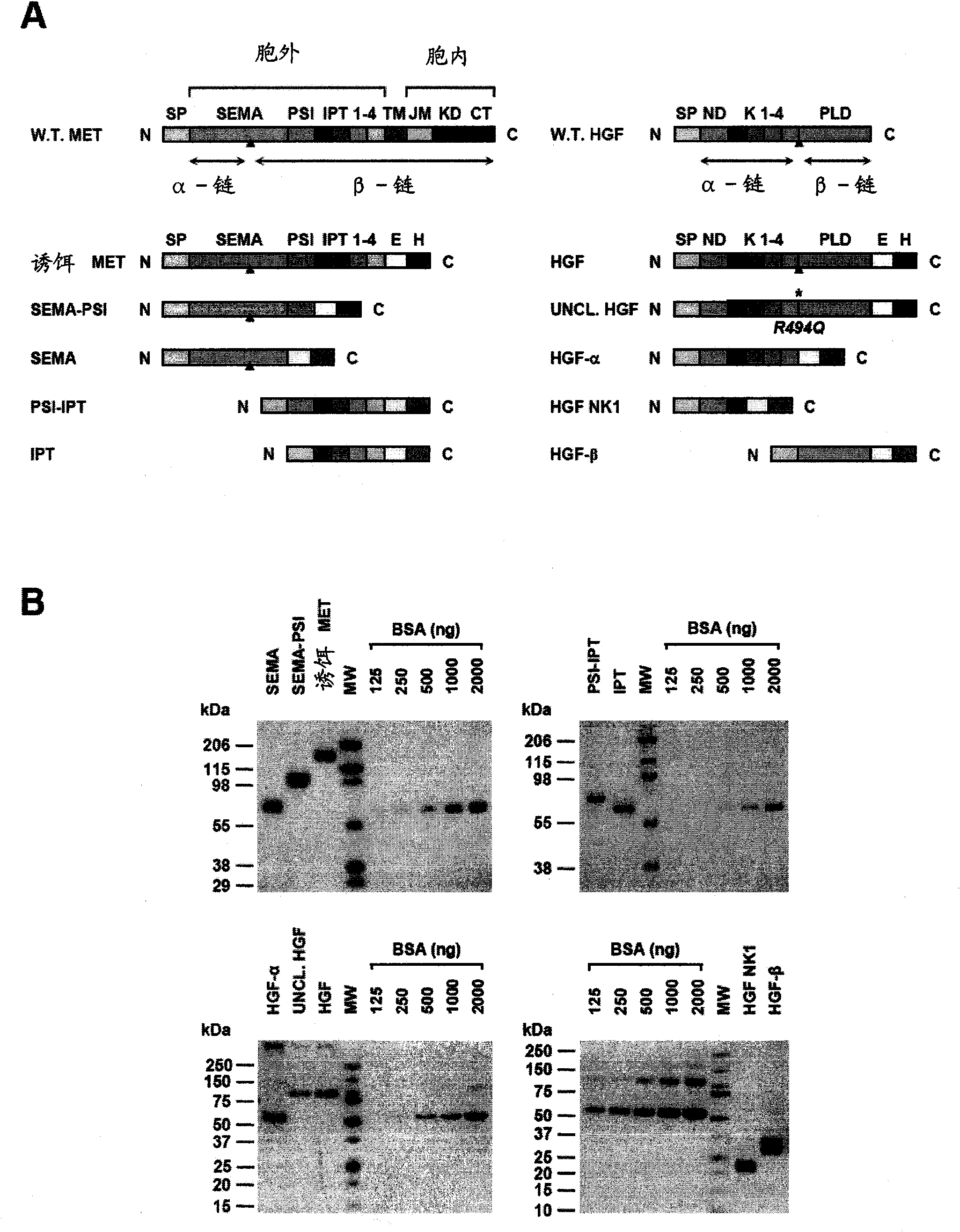
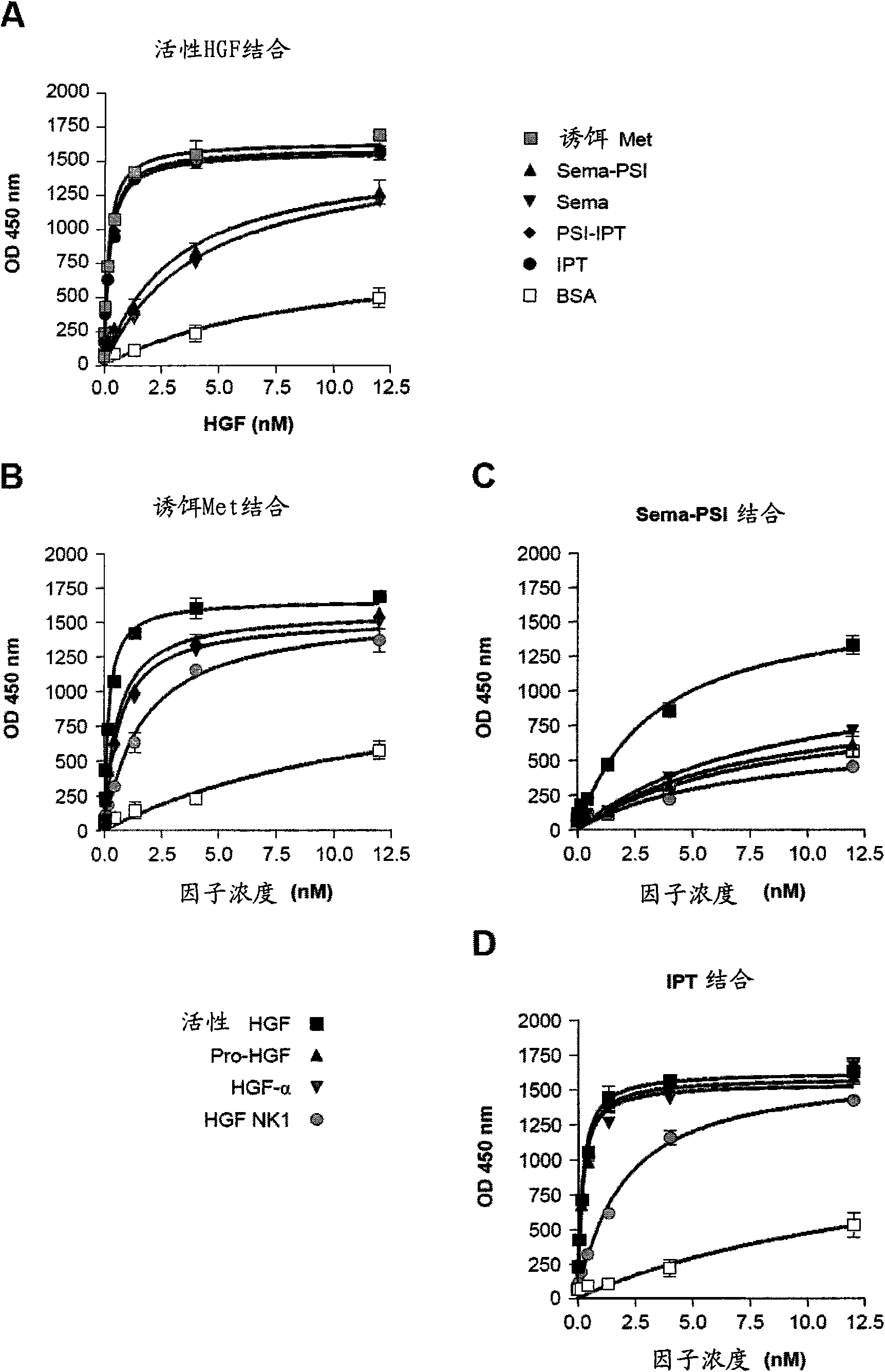
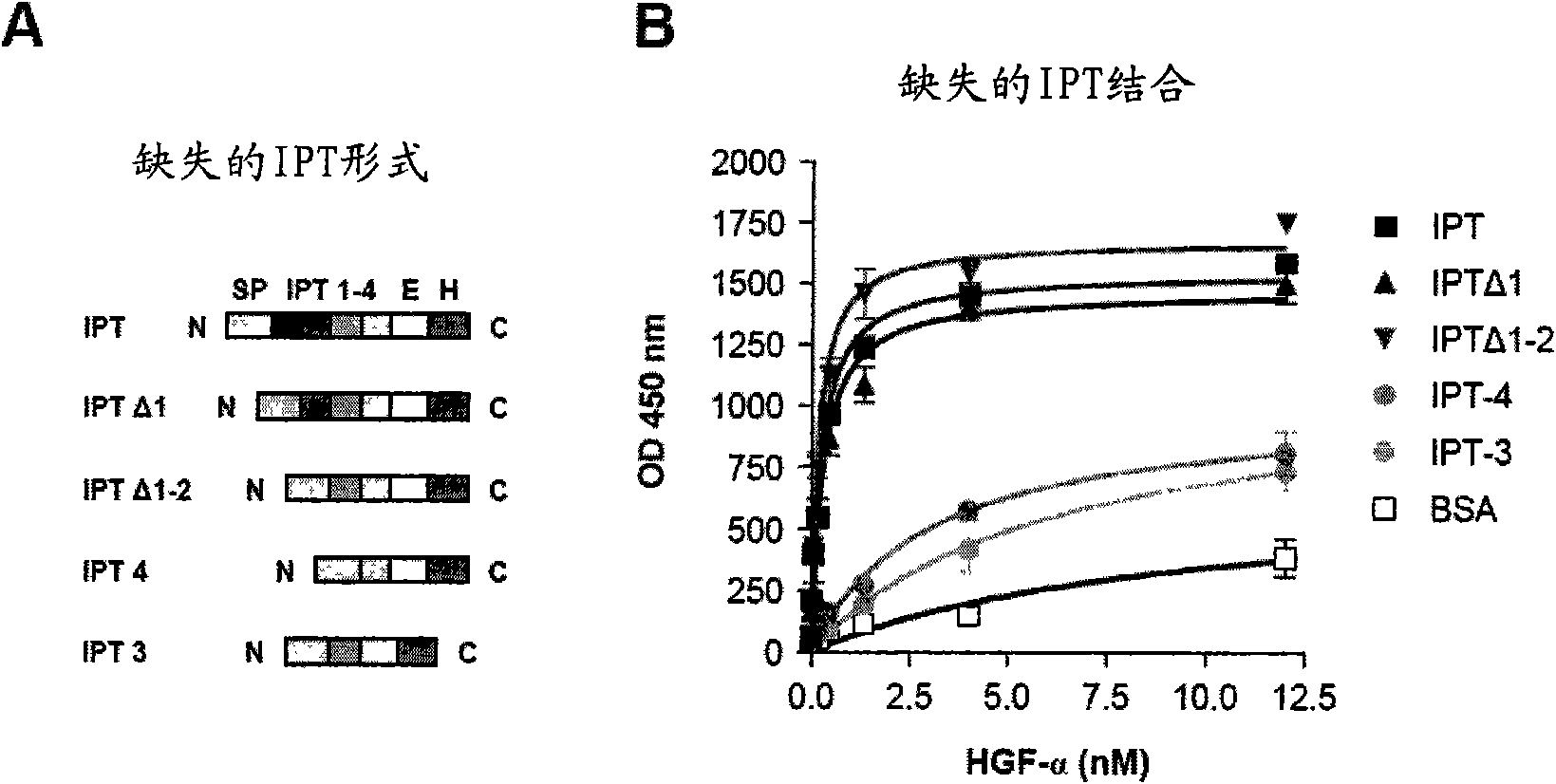
[0021] figure 1 Met and HGF subdomains are shown Genetic engineering and purification. (A) Schematic representation of the genetically engineered proteins used in this study. Left: Genetically engineered receptors. W.T.MET, wild-type Met; EXTRA, extracellular part; INTRA, intracellular part; SP, signal peptide; SEMA, semaphorin homology domain; PSI, plexin-semaphorin-integrin homology domain; IPT1- 4, Immunoglobulin-plexin-transcription factor homology domain 1-4; TM, transmembrane region; JM, membrane juxtaposition; KD, kinase domain; CT, C-terminal tail; E, FLAG or MYC epitope; H, Polyhistidine tag. Red triangles indicate the proteolytic cleavage site between the α and β chains. Right: Genetically engineered ligands. W.T.HGF, wild-type HGF; ND, N-terminal domain; K1-4, kringle 1-4; PLD, proteol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com