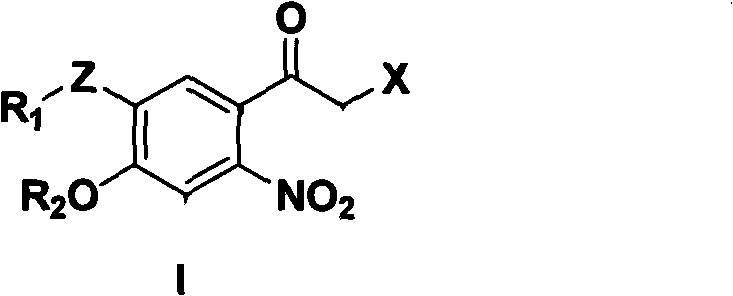
6-nitroacetophenone compound, preparation method and application thereof
A technology for nitroacetophenone and cyanoacetophenone, applied in the field of 6-nitroacetophenone compounds, can solve the problems of harsh reaction conditions, environmental pollution, adverse effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] Example 1 3-acetamido-4-ethoxyacetophenone (Compound B, R 1 = Acetyl, R 2 = Ethyl, Z = -NH-) Synthesis
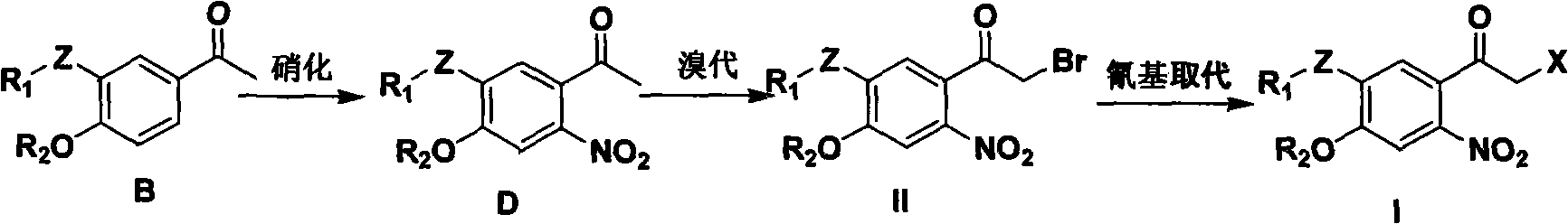
[0134] (1) Preparation of 3-nitro-4-hydroxyacetophenone
[0135] In an ice bath, add p-hydroxyacetophenone (136.2g, 1.0mol) to concentrated sulfuric acid (1000ml), stir for 10min, add potassium nitrate (96.0g, 0.95mol), stir for 2h, then add potassium nitrate (7.1 g, 0.07mol), stirred for 1h, and the reaction was over. The reaction solution was poured into 3000 ml of crushed ice to separate out a large amount of solids, filtered with suction, washed with water, and dried to obtain 171.2 g of light yellow powder of 3-nitro-4-hydroxyacetophenone with a yield of 94.5%.
[0136] (2) Preparation of 3-amino-4-hydroxyacetophenone
[0137] At room temperature, 3-nitro-4-hydroxyacetophenone (171.2g, 0.945mol) was dissolved in THF (1500ml), Raney Ni 20g was added, and the hydrogenation reaction was carried out at atmospheric pressure for 24h, and the reaction was completed. After ...
Embodiment 2
[0142] Example two 3-acetamido-4-ethoxy-6-nitroacetophenone (compound D, R 1 = Acetyl, R 2 = Ethyl, Z = -NH-) Synthesis
[0143] Compound B (R 1 = Acetyl, R 2 = Ethyl, Z = -NH-) (88.5g, 0.4mol) dissolved in nitromethane (1000ml), add fuming nitric acid (17.7ml, 0.4mol), stir at 40℃ for 12h, add fuming nitric acid (15.5ml, 0.35mol), stirring for 10h, the reaction is over. Pour the reaction solution into saturated sodium bicarbonate solution (1000ml), stir, separate, wash the organic layer with water, dry, filter, add 10g activated carbon to the filtrate, reflux for 20min, hot filter, and concentrate to obtain a red oil, 40℃ normal pressure Dry 3-acetamido-4-ethoxy-6-nitroacetophenone (compound D, R 1 = Acetyl, R 2 =ethyl, Z=-NH-) 86.5g, yield 81.2%. 1 HNMR(300MHz, CDCl 3 )δ1.52(t, 3H), 2.24(s, 3H), 2.52(s, 3H), 4.22(q, 2H), 7.53(s, 1H), 7.98(s, 1H), 8.52(s, 1H) ). ESI-MS (m / z) 265 (M-1).
Embodiment 3
[0144] Example three 3'-acetamido-4'-ethoxy-6'-nitro-2-bromoacetophenone (Compound II, R 1 = Acetyl, R 2 = Ethyl, Z = -NH-) Synthesis
[0145] The compound D(R 1 = Acetyl, R 2 = Ethyl, Z = -NH-) (79.9g, 0.3mol) dissolved in dichloromethane (1000ml), add liquid bromine (14.6ml, 0.285mol), stir for 12h at room temperature, the reaction liquid turns from light red to light Yellow, make up liquid bromine (1.1ml, 0.021mol), stir for 2h, the reaction is over. 3'-acetamido-4'-ethoxy-6'-nitro-2-bromoacetophenone (Compound II, R 1 = Acetyl, R 2 = Ethyl, Z = -NH-) 110.0g, can be used directly in the next reaction. 1 HNMR(300MHz, CDCl 3 )δ1.55(t, 3H), 2.24(s, 3H), 4.27(q, 2H), 4.30(s, 2H), 7.64(s, 1H), 8.01(s, 1H), 8.58(s, 1H) ). ESI-MS (m / z) 346 (M+1), 368 (M+23).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com