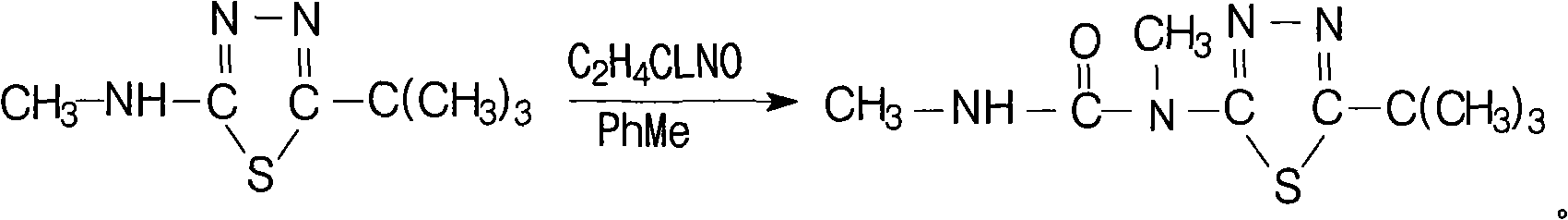
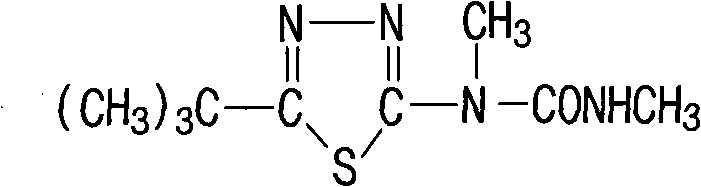
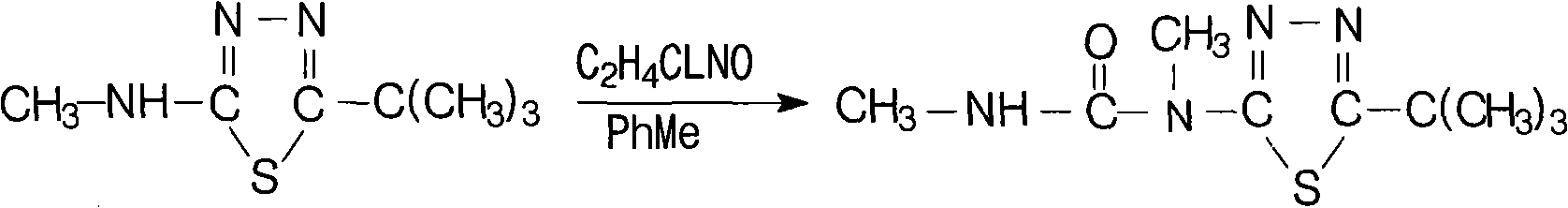
Method for synthesizing tebuthiuron technical
A technology of terbuthiauron and synthesis method, which is applied in the field of terbuthiauron technical synthesis, can solve the problems of high safety production cost, explosiveness, and inability to transport, and achieve the effects of reducing equipment investment and strong corrosiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0014] Specific example 1: 142 kg of 5-tert-butyl-2-methylamino-1,3,4 thiadiazole with a content of 95% was dissolved in 200 L of toluene to prepare a toluene solution for use. Add 85kg of carbamoyl chloride and 160L of anhydrous toluene into a 1000L reaction kettle, open the jacketed ice brine, cool down to about 20°C to 35°C, preferably at 25°C, add the above toluene solution dropwise (guarantee anhydrous), about 1.5 After the dropwise addition for 1 hour, add 82 kg of anhydrous triethylamine (99%) dropwise at 15°C to 35°C, preferably at 20°C, dropwise for about 1 hour, keep warm at 25°C for 1 hour, and heat up to 75°C- 80°C, keep warm for 4.5-5 hours, cool to below 60°C, slowly add 170kg of water (pay attention to avoid flushing), continue to cool down to 15°C-20°C, keep warm for 0.5 hours, filter, dry, and dry to a content of 97 % white product Tebuthiauron 180kg. The yield of this reaction can reach 97.0%.
specific Embodiment 2
[0015] Specific example 2: 142 g of 5-tert-butyl-2-methylamino-1,3,4 thiadiazole with a content of 95% was dissolved in 200 mL of toluene to prepare a toluene solution for use. Add 105g of carbamoyl chloride and 160mL of anhydrous toluene into a 1000mL four-neck bottle, cool down to about 20°C to 35°C, preferably 20°C, add the above toluene solution dropwise (guaranteed anhydrous), and add dropwise for about 1.5 hours After completion, add 112 g of anhydrous triethylamine (99%) dropwise at 15°C to 35°C, preferably at 25°C, dropwise for about 1 hour, keep warm at 25°C for 1 hour, and heat up to 75°C-80°C. Insulate and react for 4.5 hours, cool to below 60°C, slowly add 210g of water, continue to cool down to 15°C-20°C, keep warm for 0.5 hours, filter, dry, and dry to obtain 185g of the white product Tebuthiauron with a content of 95%. The yield of this reaction can reach 97.5%.
specific Embodiment 3
[0016] Specific Example 3: In a 1000ml four-neck bottle, add 142g of 95% 5-tert-butyl-2-methylamino-1,3,4thiadiazole at 25°C, 200ml of toluene, and 85g of methylcarbamoyl chloride. Enter nitrogen, heat up to 75°C-80°C, preferably 75°C, react for 4-5 hours, preferably 4.5 hours, cool to below 60°C, slowly add 170g of water, continue to cool down to 15°C-20°C, keep warm for 0.5 hours and then filter , dried, and dried to obtain 170 g of 92% white product Tebuthiauron. The yield of this reaction can reach 87.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com