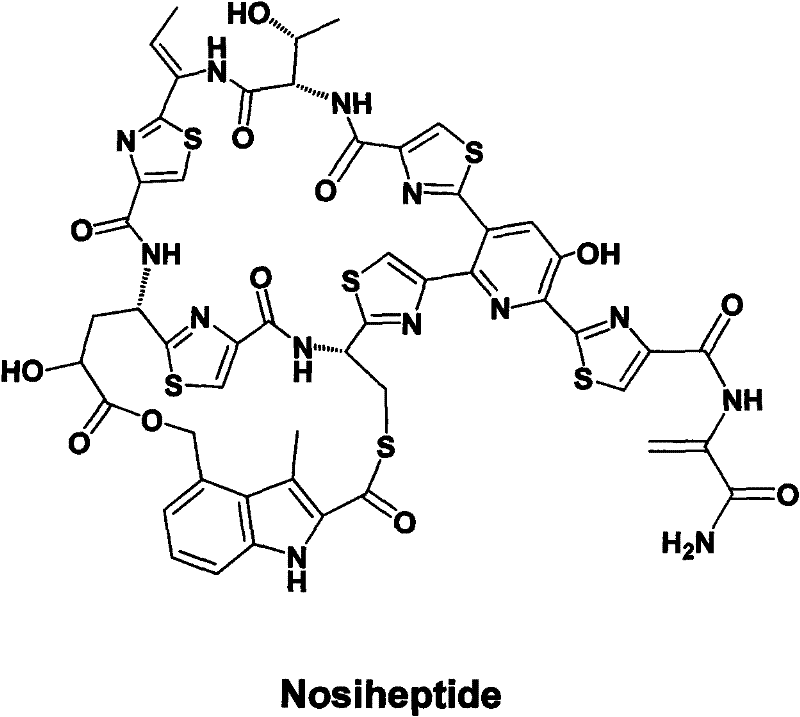
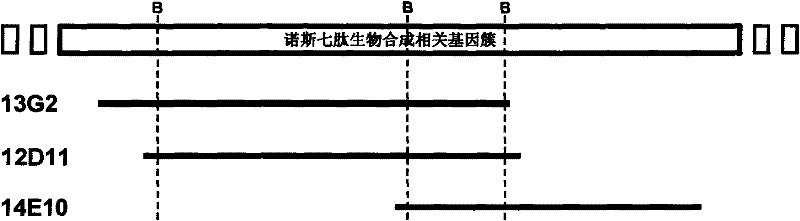
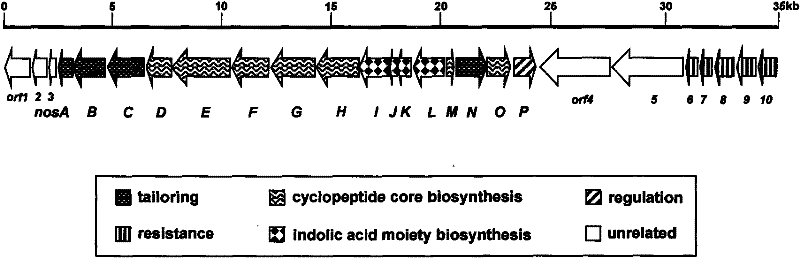
Gene cluster for biological synthesis of Nosiheptide
A biosynthesis and gene technology, applied in the field of microbial genetic resources and genetic engineering, can solve the problems of low yield, high production cost, cumbersome operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Extraction of the total DNA of Streptomyces actuosus ATCC25421, a bacterium producing heptapeptide:
[0077] 100 μL 1 x 10 8S.actuosus spore suspension was inoculated into 3mL TSB liquid medium, cultivated at 30°C, 230rpm for about 24hrs and reached the late logarithmic growth phase, then inoculated 2mL into 50mL TSB (containing 10mM magnesium chloride, 0.1% glycine), 30°C, 250rpm After cultivating for about 23 hours, it reached the early stage of the stable growth period, and it was milky yellow and turbid. The mycelium was collected by centrifuging the bacterial solution at 4°C and 3500 rpm for 15 minutes, washed with lysate, and 0.5 mL of pale milky yellow mycelium was collected. Add 10 mL of lysate (containing 5 mg / mL of lysozyme) to 1 mL of mycelium, a total of four tubes, vortex until uniform, and bathe in 37 ° C for 15 min. Add 0.1mL proteinase K (10mg / mL, freshly prepared with lysate), 1mL 10% SDS, mix well and quickly put in 70°C water bath for 15mim, it becom...
Embodiment 2
[0079] The establishment of the genetic transfer system of Streptomyces actuosus ATCC25421, which produces Nossy heptapeptide:
[0080] Culture E.coli ET12567 containing appropriate plasmids to OD 600 0.3-0.4, the bacterial cells in 30mL LB culture medium were collected by centrifugation, washed twice with an equal volume of LB, resuspended in 2mL LB, and used as E. coli donor cells. Take 500 μL of 20% glycerol spore suspension of Streptomyces actuosus ATCC25421 frozen at -80°C, wash twice with an equal volume of TES buffer (50 mM TES Na, pH 8.0), resuspend in an equal volume of TES buffer, Heat shock at 50°C for 10 min to germinate the spores. Add an equal volume of TSB medium and incubate at 37°C for 2-5hr. Centrifuge and resuspend in 0.5-1 mL LB as Streptomyces recipient cells. Mix 100 μL of different concentrations of recipient cells with an equal volume of donor cells and directly spread it on a medium containing 10 mM MgCl 2 After incubating at 30°C for 20 hours, gen...
Embodiment 3
[0083] Construction of Genome Library of Streptomyces actuosus ATCC25421 Genome Library
[0084] Firstly, the total DNA of Streptomyces actuosus ATCC25421 was sucked several times with a 1ml syringe, so that the total DNA was randomly broken, and the DNA was subjected to gradient centrifugation using sucrose gradient centrifugation, and the DNA fragments slightly larger than 40kb were collected, dephosphorized, and set aside. pJTU2554 was first cut from the middle of the two cos sequences with EcoRV, and ligated with the prepared 40kb DNA fragment overnight. Thaw the PromegaPackagene extract stored at -80°C on ice, immediately add 10ul of the ligation product, mix well by flicking, and place it at room temperature (about 22°C) for 3hr. Add 445ul Phage buffer, invert to mix; add 25ul chloroform to terminate the reaction, centrifuge to make the chloroform sink to the bottom, and store at 4°C. The strain E.coli LE392 frozen at -80°C was spread on LB medium for recovery. Take a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com