Solid acid catalyst, preparation thereof and application thereof in esterification reaction
A technology of solid acid catalyst and alkyl sultone, applied in catalyst activation/preparation, carboxylate preparation, organic compound preparation and other directions, to achieve the effect of convenient operation, simple synthesis process and clean process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] [Example 1] Synthesis of Arylated Phosphotungsto Heteropoly Salt A:
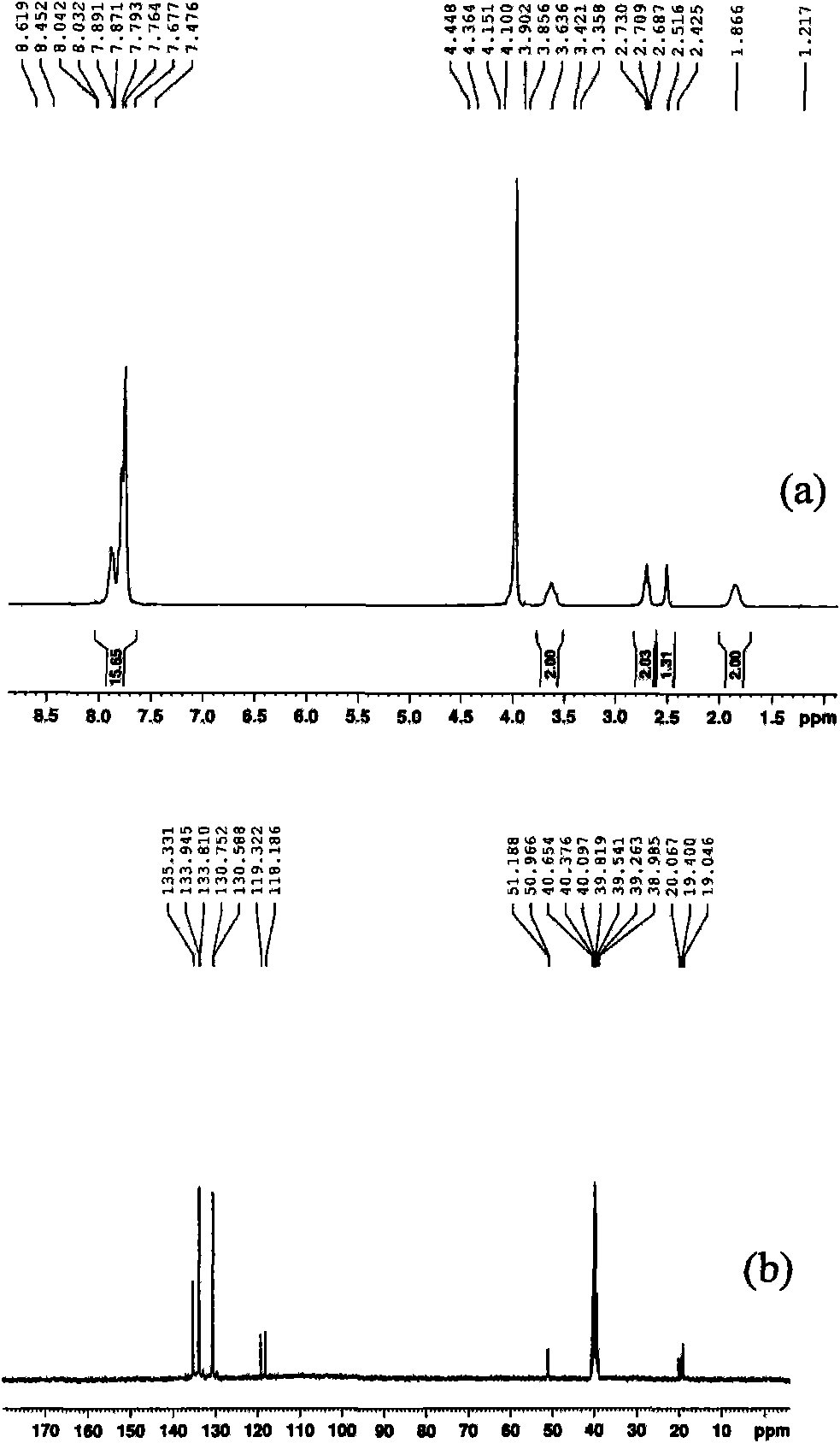
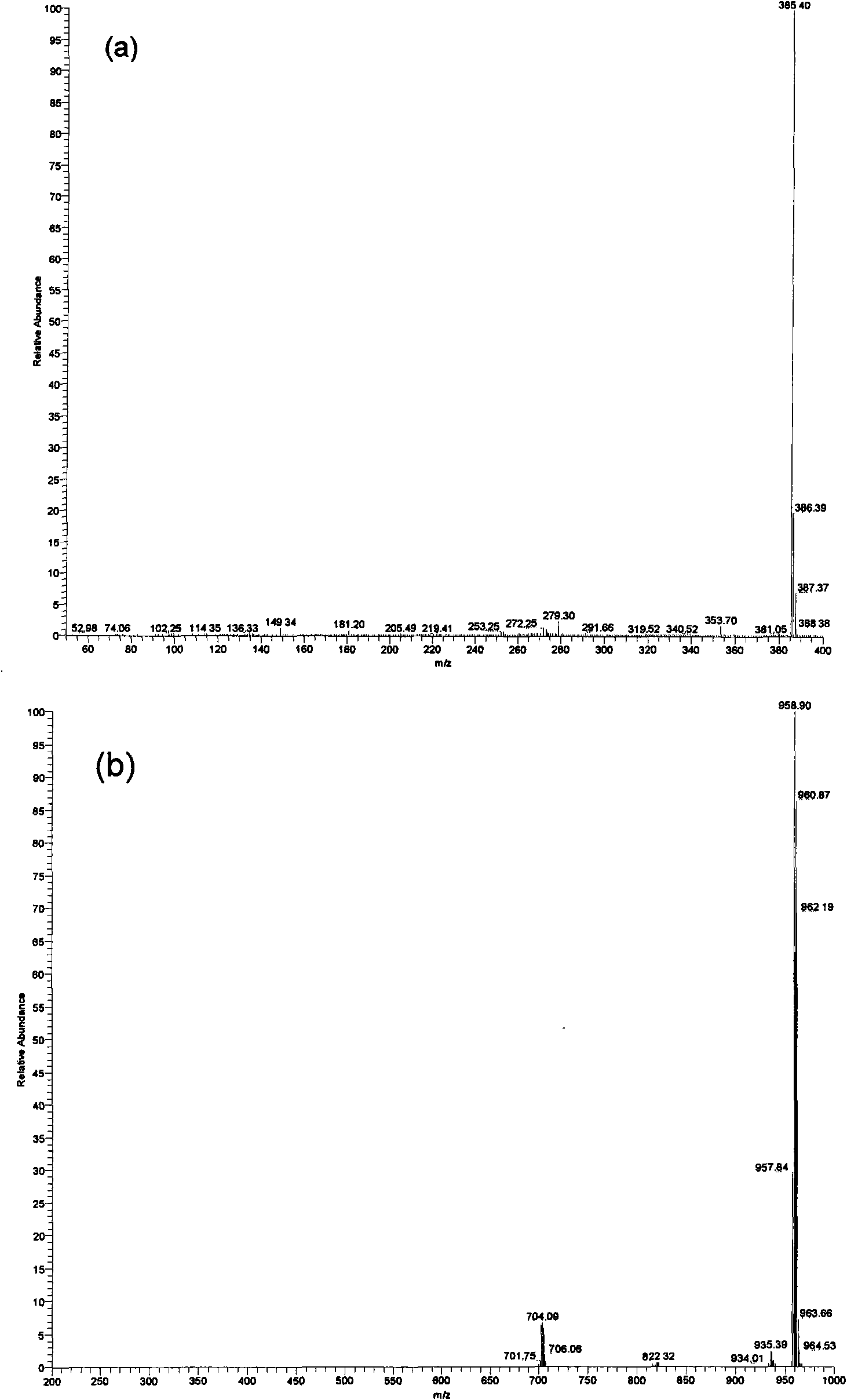
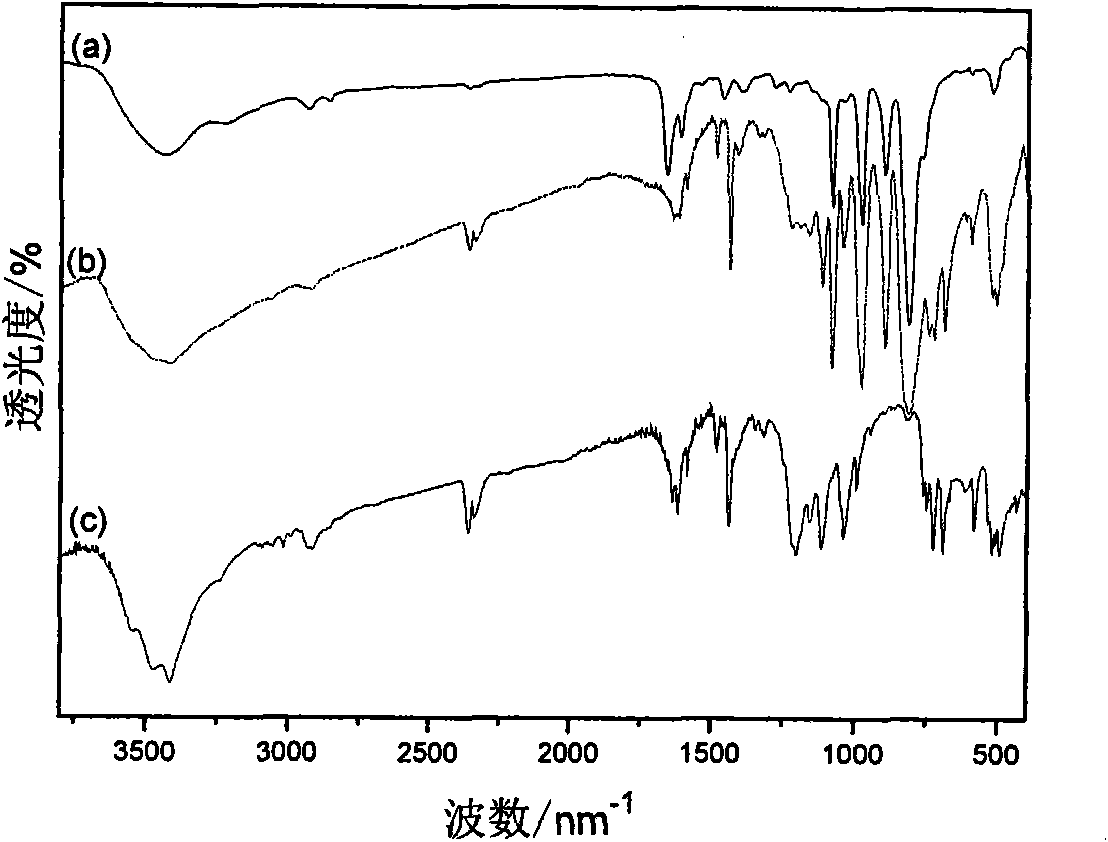
[0027] (1) In a 100 mL three-necked flask equipped with a magnetic stirring bar, a constant-pressure dropping funnel and a spherical condenser, add 5.25 g of triphenylphosphine and 20 mL of toluene, stir to dissolve, and heat up to 40℃, digital temperature controller controls the temperature. 2.69g of 1,3-propane sultone and 15mL of toluene were prepared into a solution and added dropwise to the above solution through a constant pressure dropping funnel. After the dropwise addition was completed for 2 hours, the solution was stirred at 40 °C for 24 hours, and then heated to 140 °C and stirred 36h. Suction filtration, repeatedly washed with toluene, and dried at 100 °C for 12 h to obtain 6.73 g of 3-sulfonic acid propyltriphenylphosphine inner salt (C 6 H 5 ) 3 P(CH 2 ) 3 SO 3 H, 87.5% yield.
[0028] (2) In a 250 mL single-necked flask equipped with a magnetic stirring bar and a constant-press...
Embodiment 2
[0030] [Example 2] Synthesis of butylsulfonic acid functionalized arylphosphotungstic heteropoly acid salt B:
[0031] (1) In a 100 mL three-necked flask equipped with a magnetic stirrer, a constant-pressure dropping funnel and a spherical condenser, add 2.62 g of triphenylphosphine and 10 mL of toluene, stir to dissolve, and heat up to 40℃, digital temperature controller controls the temperature. 1.36g of 1,4-butanesultone and 15mL of toluene were prepared into a solution and added dropwise to the above solution through a constant pressure dropping funnel. The dropwise addition was completed for 2 hours, and then heated to 140°C and stirred for 48 hours. Suction filtration, washed repeatedly with toluene, and dried at 100 °C for 12 h to obtain 3.54 g of 4-sulfonic acid butyltriphenylphosphine inner salt (C 6 H 5 ) 3 P(CH 2 ) 4 SO 3 H, 88.9% yield.
[0032] (2) In a 250 mL single-necked flask equipped with a magnetic stirring bar and a constant-pressure dropping funnel,...
Embodiment 3
[0033] [Example 3] Synthesis of alkylated phosphotungstic heteropoly acid salt C:
[0034] (1) In a 100 mL three-necked flask equipped with a magnetic stirring bar, a constant-pressure dropping funnel and a spherical condenser, add 1.22 g of 1,3-propane sultone and 10 mL of benzene. While stirring, a solution of 0.76 g of trimethylphosphine and 10 mL of benzene was added dropwise to the above solution through a constant pressure dropping funnel. Suction filtration, repeatedly washed with benzene, and dried at 100 °C for 12 h to obtain 1.56 g of 3-sulfonic acid propyltrimethylphosphine inner salt (CH 3 ) 3 P(CH 2 ) 3 SO 3 H, 78.8% yield.
[0035] (2) In a 250 mL single-necked flask equipped with a magnetic stirring bar and a constant-pressure dropping funnel, add 0.4 g of 3-sulfonic acid propyltrimethylphosphine inner salt and 50 mL of water, and stir to dissolve. A solution prepared from 2.1 g of 12-phosphotungstic acid and 50 mL of water was added dropwise to the above ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com