Application of near infrared fluorescent chemical in lymphaden imaging and angiogram
A fluorescent compound and angiography technology, applied in the field of medical in vivo imaging, can solve the problems of difficult degradation, metabolism, toxicity, etc., achieve good photostability, high quantum yield, and make up for the effect of short coloring time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to Embodiment 7
[0052] Particularly preferred compounds provided by the present invention are shown in Table 1:
[0053] Table 1: The structural formula of the compound of the preferred embodiment of the present invention
[0054]
[0055]
[0056]
[0057] The R2 group in the near-infrared fluorescent compound formula I is hydrogen, alkyl (for example, methane group, ethyl group etc.), aryl group (for example, phenyl, anthracenyl etc.), aralkyl group (for example, tolyl or ethylphenyl), alkyl sulfonates (for example, sodium butane sulfonate or sodium pentane sulfonate), alkyl carbonates (for example, butane sodium carbonate, pentane potassium carbonate, etc.), alkylamines ( For example, groups such as butanylamine, propanylamine, etc.), carboxyl also have the effects described in the present invention; the X group in the compound adopts iodine or chlorine or bromine or sulfurized alkyl (for example, OSO 2 -Methyl, OSO 2 -ethyl) or BF 4 or ClO 4 , R1 group adopts chlorine or brom...
Embodiment 9
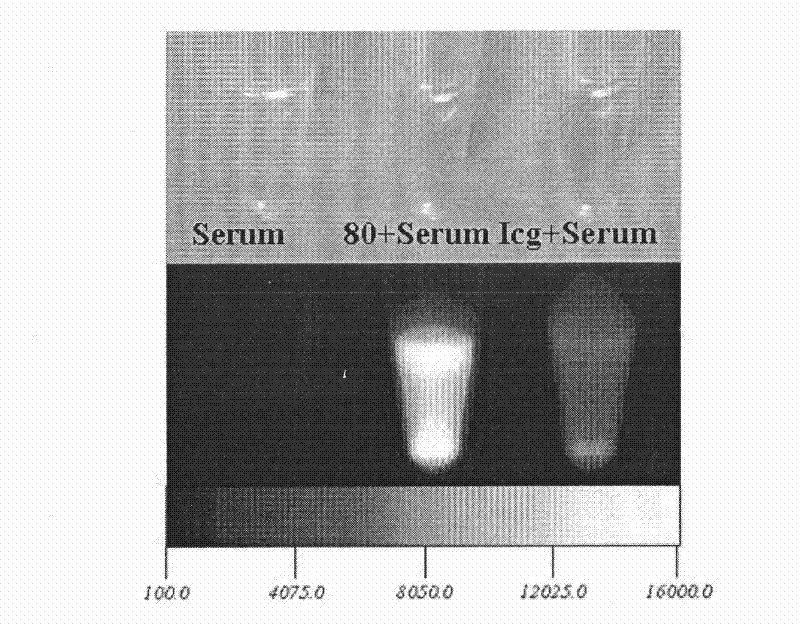
[0059] With 100% bovine serum as blank control, compound 1 or 2 or 7 described in Table 1 was added to bovine serum, and the final concentration of the resulting solution was 50ummol / L; in addition, indocyanine green (ICG) was added to bovine In serum, the final concentration of the resulting solution was 50ummol / L, and then carried out near-infrared fluorescence imaging respectively. It can be seen that the fluorescence intensity of compound 1 or 2 or 7 of the present invention in serum is significantly higher than that of ICG, and the fluorescence intensity when the two are dissolved in water similar. (See figure 1 , where the left 1 is blank serum, the middle is the addition of compound 1 or 2 or 7 of the present invention to the serum, and the right 1 is the addition of indocyanine green to the serum)
Embodiment 10
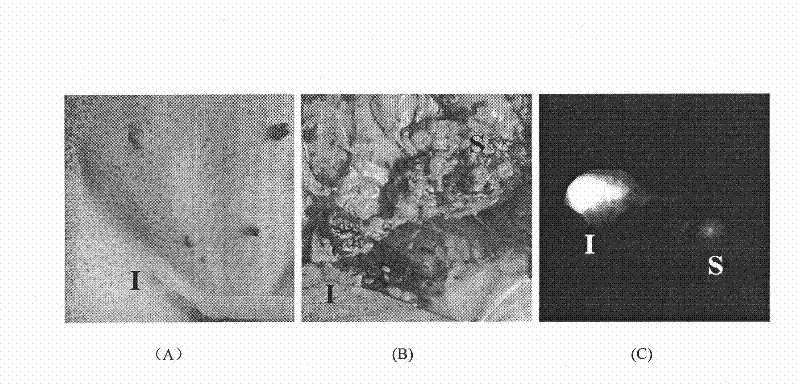
[0061] Take 1 Kunming female mouse of about 25 g, anesthetize it with 1% pentobarbital sodium solution, shave off the body hair, take the side lying position, and take 10 ul of the compound 1 or 2 of the present invention with a concentration of 50 μmol / L or 7. Insert the needle between the second finger of the mouse’s forefoot, inject subcutaneously and gently massage the injection site. 5-30 minutes after injection, observe in the Kodak in vivo imager. The excitation wavelength is 770nm, the emission wavelength is 830nm, and the exposure time is 20 seconds. .
[0062] Results: High fluorescent signal was detected in the lymph nodes near the axilla of the mouse, which was in obvious contrast with the surrounding tissue. The high signal of the lymph nodes could still be seen after 3 hours of observation. The same site was injected with 1% methylene blue as a control, and it was confirmed that this site was indeed the draining sentinel lymph node of the injection site. After l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com