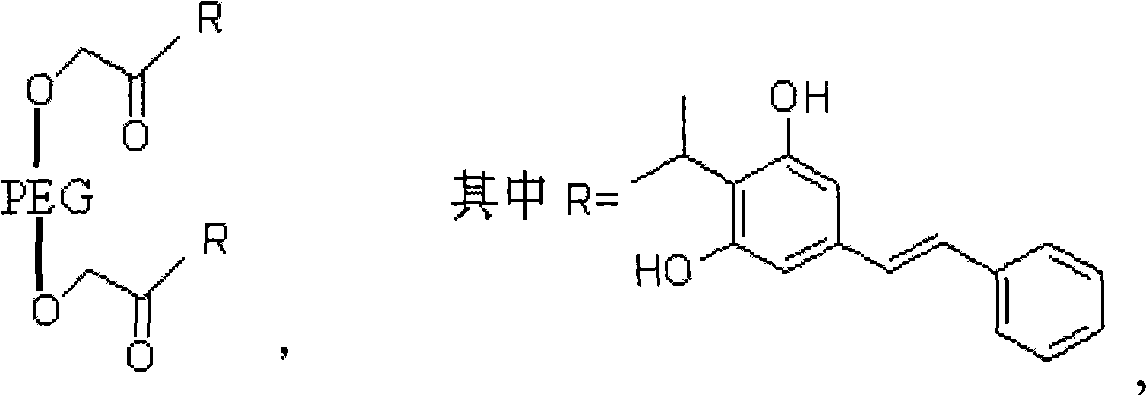
3, 5-dihydroxy-4-isopropyl diphenyl ethylene-ethyl bromoacetate-polyoxyethylene compound and synthetic method thereof
A technology of isopropyl stilbene and hydroxy stilbene, which is applied in the field of prodrugs of antifungal drugs, can solve the problems of inability to maintain effective blood drug concentration, low absolute bioavailability, and short biological half-life, and achieve improved Bioavailability, good water solubility, and the effect of prolonging the half-life of the drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
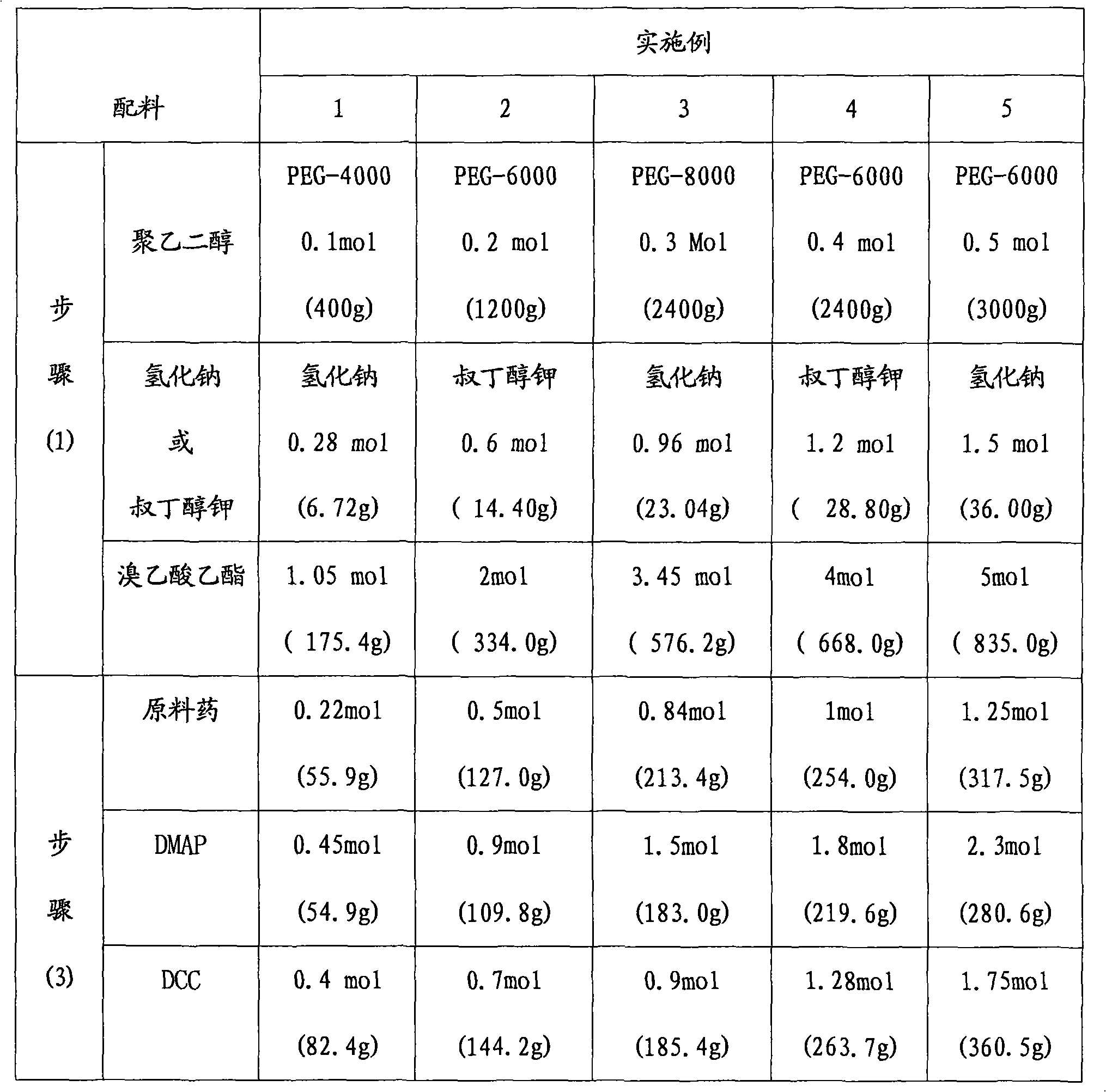
Examples
Embodiment Construction
[0024]
[0025] The synthetic route of embodiment 1-embodiment 5 is as follows:
[0026]
[0027] in
[0028] Its synthetic method is carried out according to the following step order:
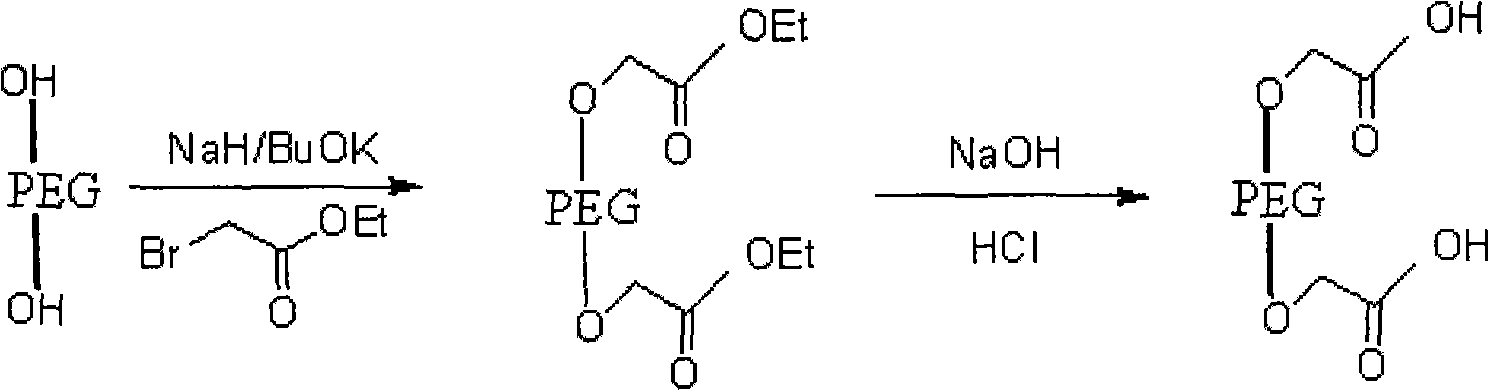
[0029] (1) Preparation of polyethylene glycol-ethyl diacetate
[0030] Its reaction formula is as follows:
[0031]
[0032] The operation process is as follows: take dry polyethylene glycol, dissolve in toluene, add sodium hydride or potassium tert-butoxide at a molar ratio of 1:3, stir at room temperature, add ethyl bromoacetate, and reflux for 22-25 hours; evaporate under reduced pressure Toluene was extracted with dichloromethane, concentrated under reduced pressure, anhydrous ether was added, and a white solid A was precipitated, namely polyethylene glycol-ethyl diacetate;
[0033] (2) Polyethylene glycol-diacetate hydrolysis prepares polyethylene glycol-diacetate
[0034] The reaction formula is as follows:
[0035]
[0036] The operation process is as follows: Take ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com