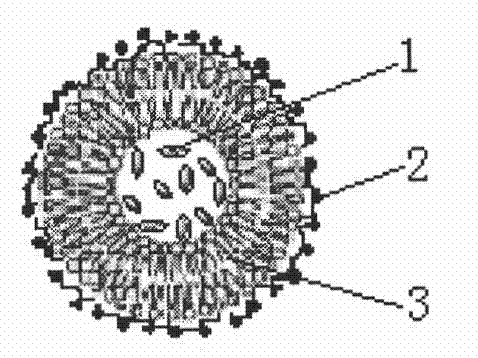
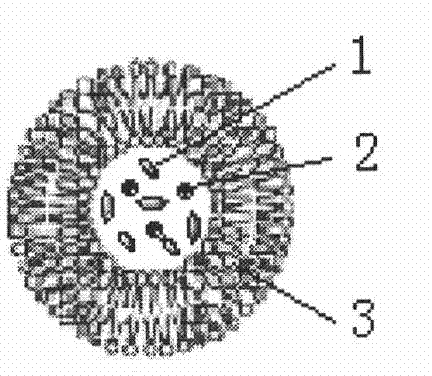
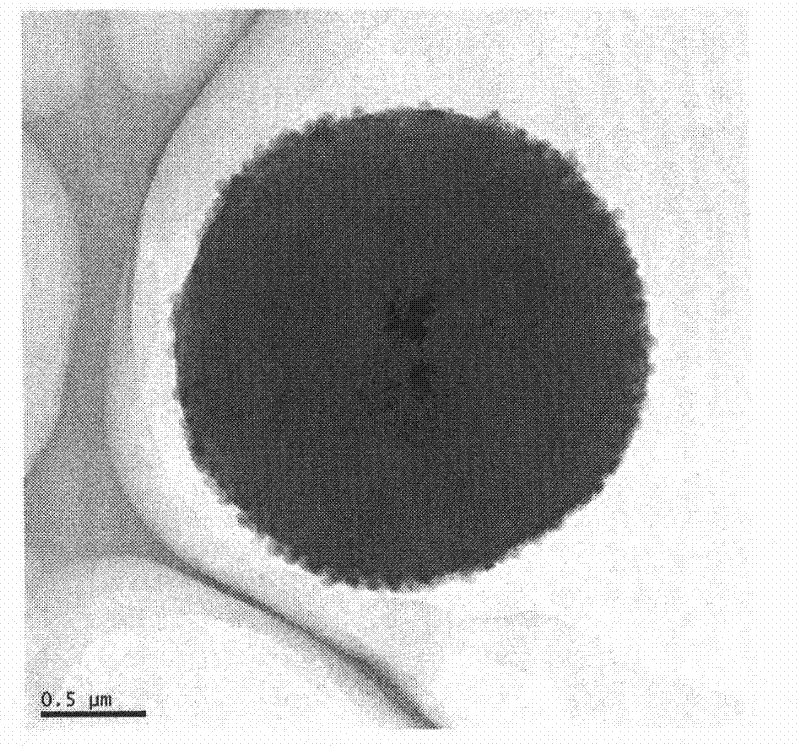
Method for preparing magnetic liposome
A magnetic liposome and liposome technology, which are applied in the directions of liposome delivery, medical preparations of inactive ingredients, pharmaceutical formulations, etc., to achieve the effects of increasing the encapsulation capacity and increasing the magnetic content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Egg yolk lecithin and cholesterol were dissolved in methanol-chloroform mixed organic solvent (1:2, v / v) respectively to obtain 0.1g / mL egg yolk lecithin solution and 0.1g / mL cholesterol solution, and 10mL of egg yolk lecithin solution and 2.5mL of cholesterol solution were mixed in an eggplant-shaped bottle, and rotovaped to form a liposome film. Add 50mL of phosphate buffer solution (pH, 7.4) into the eggplant-shaped bottle forming the liposome film, use a vortex mixer to vortex for 20min, use a high-speed dispersing homogenizer to disperse for 5min, and centrifuge and wash twice with water after fully hydrating to obtain a surface Negatively charged liposomes. Disperse 1 g of the above-mentioned negatively charged liposomes in 60 mL of polydiallyldimethylammonium chloride aqueous solution with a concentration of 0.8 mg / (ml 0.5 M NaCl), let stand for adsorption for 20 min, and then centrifuge and wash with water; redisperse In 60mL of polystyrene sodium su...
Embodiment 2
[0025] Embodiment 2: Egg yolk lecithin and cholesterol are dissolved in methanol-chloroform mixed organic solvent (1: 2, v / v) respectively to obtain 0.1g / mL egg yolk lecithin solution and 0.1g / mL cholesterol solution. 10mL of egg yolk lecithin solution and 2mL of cholesterol solution were mixed in an eggplant-shaped bottle, and the liposome film was formed by rotary evaporation. Add 60mL of phosphate buffer solution (pH, 7.4) into the eggplant-shaped bottle forming the liposome film, use a vortex mixer to vortex for 30min, use a high-speed dispersing homogenizer to disperse for 6min, and centrifuge and wash 3 times with water after fully hydrating to obtain a surface Negatively charged liposomes. Disperse 1 g of the above-mentioned negatively charged liposomes on the surface in 70 mL of polydiallyldimethylammonium chloride aqueous solution with a concentration of 1 mg / (ml 0.5 M NaCl), let stand for adsorption for 20 min, and then wash with centrifugal water; then disperse in ...
Embodiment 3
[0026] Example 3: Egg yolk lecithin and cholesterol were dissolved in methanol-chloroform mixed organic solvent (1:2, v / v) respectively to obtain 0.1g / mL egg yolk lecithin solution and 0.1g / mL cholesterol solution, and 10mL of egg yolk lecithin solution and 2mL of cholesterol solution were mixed in an eggplant-shaped bottle, and the liposome film was formed by rotary evaporation. Add 70mL of phosphate buffer solution (pH, 7.4) into the eggplant-shaped bottle forming the liposome film, use a vortex mixer to vortex for 30min, use a high-speed dispersing homogenizer to disperse for 8min, centrifuge and wash twice with water after fully hydrating to obtain a surface Negatively charged liposomes. Disperse 1 g of the above-mentioned negatively charged liposomes on the surface in 70 mL of polydiallyldimethylammonium chloride aqueous solution with a concentration of 1.2 mg / (ml 0.5 M NaCl), let stand for adsorption for 30 min, then centrifuge and wash with water; redisperse In 70mL of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com