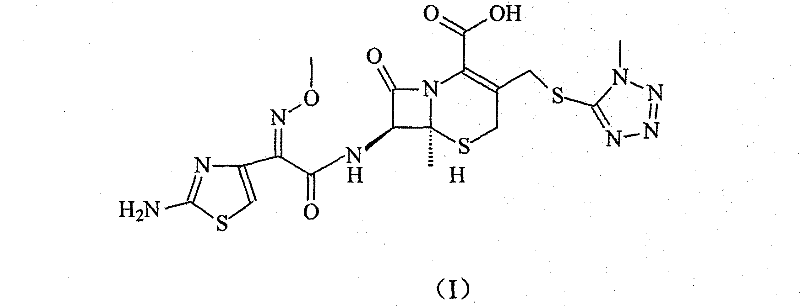
Cefmenoxime compound and synthetic method thereof
A synthetic method, cefmenoxime technology, applied in the field of drug synthesis, can solve problems such as no literature and patent reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
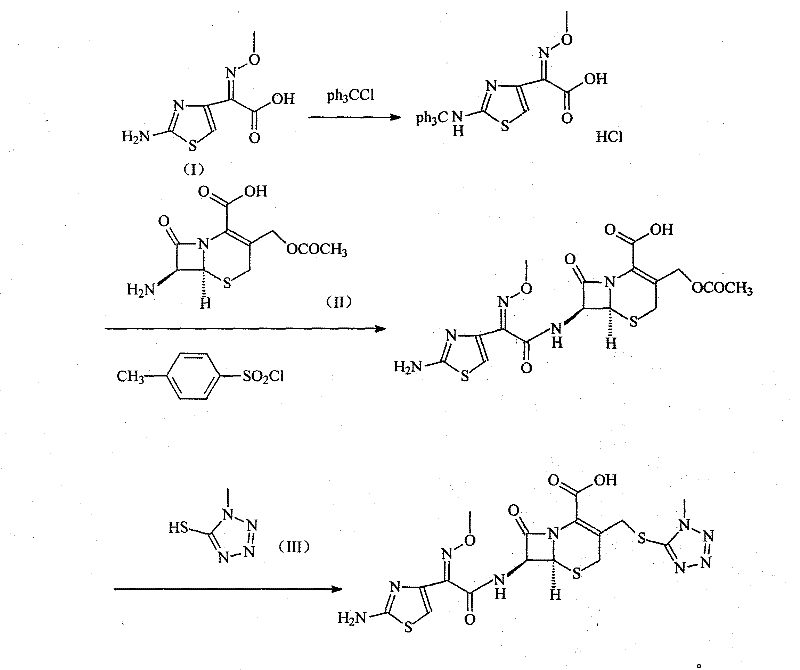
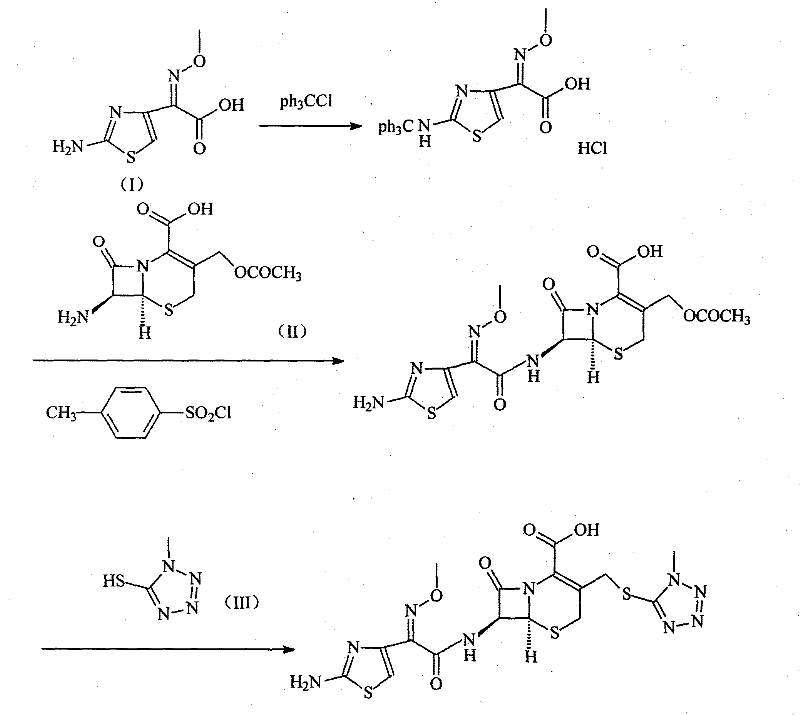
[0030] Synthesis of embodiment 12-(2-tritylaminothiazol-4-yl)-2-methoxyiminoacetic acid hydrochloride
[0031] The 2-(2-aminothiazol-4-yl)-2-methoxyiminoacetic acid of 201 grams and the triphenylchloromethane of 280 grams are joined in the DMF of 600ml, reacted at room temperature for 4 hours, then slowly added 1 Liter diisopropyl ether, stirred, precipitated solid, filtered, washed with a small amount of diisopropyl ether, and dried to obtain 460 g of the product, with a yield of 96%.
Embodiment 2
[0032] The synthesis of embodiment 2 cefotaxime hydrochloride
[0033] 326 grams of 2-(2-tritylaminothiazol-4-yl)-2-methoxyiminoacetic acid hydrochloride and 120 ml of N,N-diisopropylethylamine were added to 500 ml of DMF In, reactant is cooled to 10 ℃, add 130 grams (0.68mol) p-toluenesulfonyl chloride, stir reaction at this temperature for 1 hour, then add 185 grams (0.68mol) 7-ACA and 300ml triethylamine, in 5 Stir vigorously at -10°C for 0.5 hours, then add 500ml of 6mol / L hydrochloric acid, raise the temperature to 45°C, stir for 1.5 hours, cool to room temperature, then add 4 liters of acetone and 300ml of water and stir at room temperature to precipitate a solid, filter, Wash with acetone and dry under vacuum at 40° C. to obtain 314 g of product, yield: 94%.
Embodiment 3
[0034] The synthesis of embodiment 3 cefmenoxime
[0035] Dissolve 200 grams of cefotaxime hydrochloride in a mixed solvent of 5000 ml of distilled water and 2500 ml of acetone, add 47.2 grams of 1-methyl-5-mercapto-1H-tetrazolium, stir and heat up to 55 ° C, and then use 3% carbonic acid Sodium aqueous solution adjusts the pH of the reaction system to 6.5-7, reacts at this temperature for 4 hours, adds activated carbon to decolorize, then cools to room temperature, filters, and the filtrate is adjusted to PH=6 with 2mol / L hydrochloric acid, and 500ml of ethyl acetate The ester was extracted twice, and the water phase was adjusted to PH=2.5 with 2 mol / L hydrochloric acid, the solid was precipitated by stirring, filtered, the filter cake was washed with water, and dried to obtain 202 g of the product, with a yield of 93.7%.
[0036] Product properties: off-white crystalline powder.
[0037] Purity: 99.4%.
[0038] Elemental Analysis C 16 h 17 N 9 o 5 S 3 , molecular weig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com