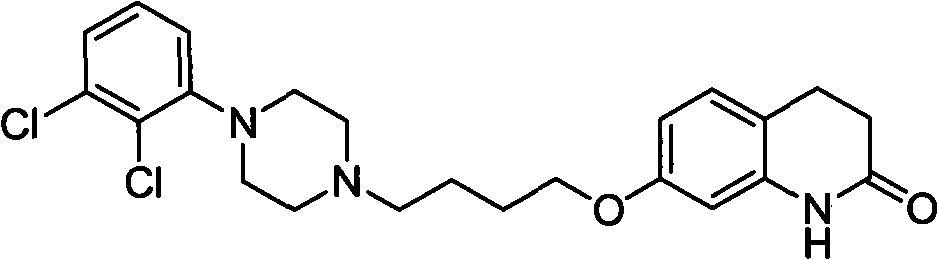
Novel preparation method and intermediate for aripiprazole
A general formula and compound technology, applied in the field of drug synthesis, can solve the problems of unfavorable industrialized large-scale production, harsh reaction conditions, and poor reaction selectivity, and achieve the effects of simple operation, high selectivity, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
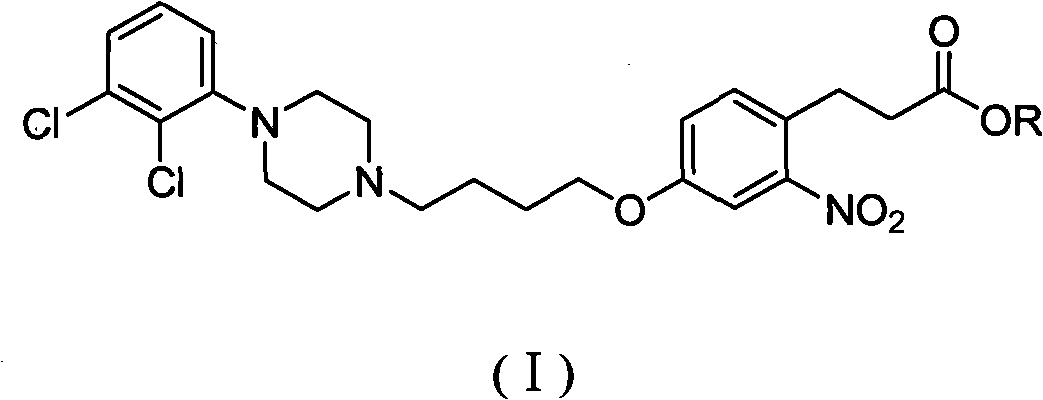
[0044] Embodiment 1 general formula (I) intermediate 3-(4-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-nitrophenyl)propyl Preparation of ethyl acetate
[0045] Step 1: Put 4-methyl-3-nitrophenol VIII (90.0g, 0.588mol) in a 500mL round bottom flask, add acetic anhydride (66.7mL, 0.706mol) and 4-N,N-di Aminopyridine (3.6g, 0.029mol), react at room temperature for 1 hour, then add drinking water (300mL), stir for 1 hour, filter, wash the filter cake with drinking water until the filtrate is neutral, drain the filter cake, and then It was dried at constant temperature for 3 hours at °C, and then dried in vacuum for 24 hours to give 4-methyl-3-nitrophenyl acetate VII (112 g, 97.6%) as a white solid, mp 68.4-70.1 °C.
[0046] Step 2 Put the product 4-methyl-3-nitrophenyl acetate VII (112g, 0.573mol) obtained in step 1 into a 1000mL three-necked flask, add carbon tetrachloride (450mL), stir until dissolved, and then Add N-bromosuccinimide (112.5 g, 0.631 mol) and benzoyl pero...
Embodiment 2
[0057] Example 2 General formula (I) intermediate 3-(4-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-nitrophenyl) Preparation of ethyl propionate
[0058] Step 1, put 4-methyl-3-nitrophenol VIII (100g, 0.653mol) in a 1000mL round bottom flask, add acetic anhydride (75mL, 0.784mol) and 4-N,N-dimethylamino under stirring Pyridine (3.98 g, 0.033 mol), acetic acid (50 mL). After reacting at room temperature for 2 hours, slowly add drinking water (600mL) to the reaction bottle, stir for 1 hour, filter, wash the filter cake with drinking water until the filtrate is neutral, drain the filter cake, and then dry at 40°C for 3 hours. Drying in vacuo for 24 h afforded 4-methyl-3-nitrophenylacetate VII (121 g, 95%) as a white solid, mp 68.4-70.1°C.
[0059] Step 2, put the product 4-methyl-3-nitrophenyl acetate VII (27.8g, 0.142mol) obtained in step 1 into a 500mL three-necked flask, add carbon tetrachloride (180mL), and stir until dissolved , then add N-bromosuccinimide (30.5g, 0...
Embodiment 3
[0068] Example three general formula (I) intermediate 3-(4-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-2-nitrophenyl) Preparation of ethyl propionate
[0069] Steps 1, 2, 4, 5, 6, and 7 can refer to the corresponding steps of Embodiment 1 or Embodiment 2 respectively, and Step 3 is replaced by:
[0070] Diethyl malonate (25.67g, 0.160mol) was placed in a 500mL three-necked flask, and toluene (180mL) was added, and the reaction solution was lowered to about 0°C in an ice-water bath while stirring, and sodium tert-butoxide ( 19.8g, 0.206mol), stirred for 0.5 hours after adding, added dropwise the toluene (40mL) solution of yellow solid product VI (31.4g, 0.115mol) obtained in step 2, stirred at room temperature for 3 hours after adding, then added drinking water ( 100ml), extracted with toluene (70ml×3), dried over magnesium sulfate and spin-dried to obtain yellow liquid product 2-(4-acetoxy-2-nitrobenzyl)diethyl malonate V (38.8g, 95.9 %).
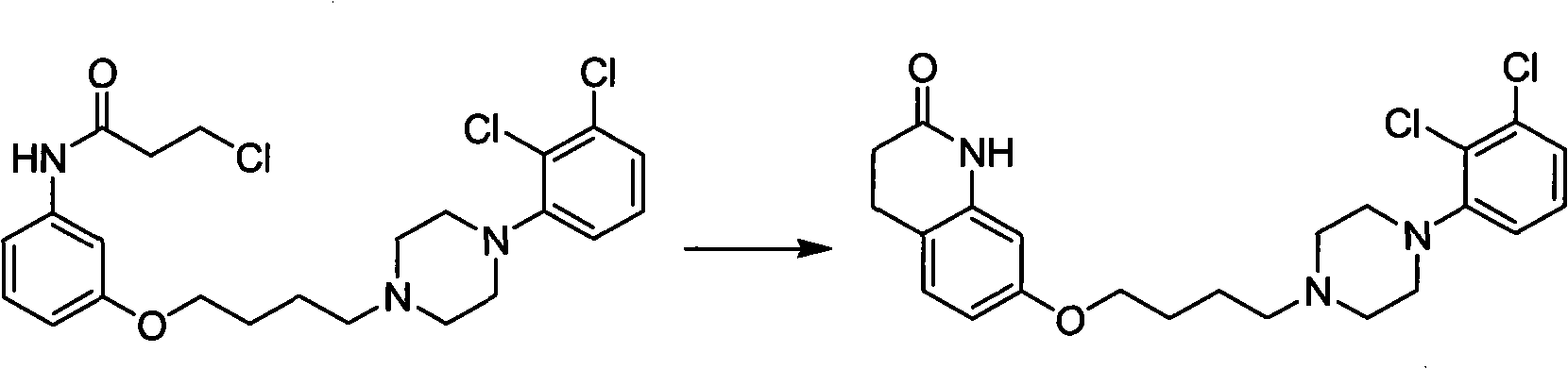
[0071] Embodiment four uses ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com